Question: thermodynamics question, step by step pls The molar enthalpy for a mixture of toluene (1) + oxygen (2) at 298.15K and 1 bar is given
thermodynamics question, step by step pls 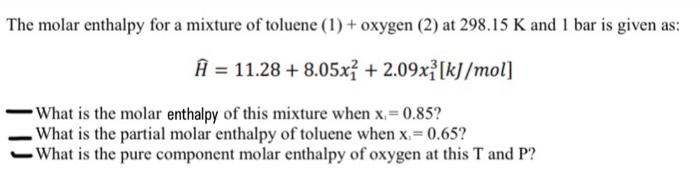
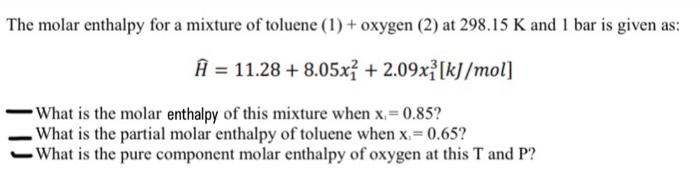
The molar enthalpy for a mixture of toluene (1) + oxygen (2) at 298.15K and 1 bar is given as: H=11.28+8.05x12+2.09x13[kJ/mol] What is the molar enthalpy of this mixture when xi=0.85 ? What is the partial molar enthalpy of toluene when x1=0.65 ? What is the pure component molar enthalpy of oxygen at this T and P ? The molar enthalpy for a mixture of toluene (1) + oxygen (2) at 298.15K and 1 bar is given as: H=11.28+8.05x12+2.09x13[kJ/mol] What is the molar enthalpy of this mixture when xi=0.85 ? What is the partial molar enthalpy of toluene when x1=0.65 ? What is the pure component molar enthalpy of oxygen at this T and P
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


