Question: This is a Physical Chemistry 2 question, and not general chemistry. Please answer parts a, b, and c. 1. For propionic acid Ka=1.34105 at 25C.
 This is a Physical Chemistry 2 question, and not general chemistry. Please answer parts a, b, and c.
This is a Physical Chemistry 2 question, and not general chemistry. Please answer parts a, b, and c.
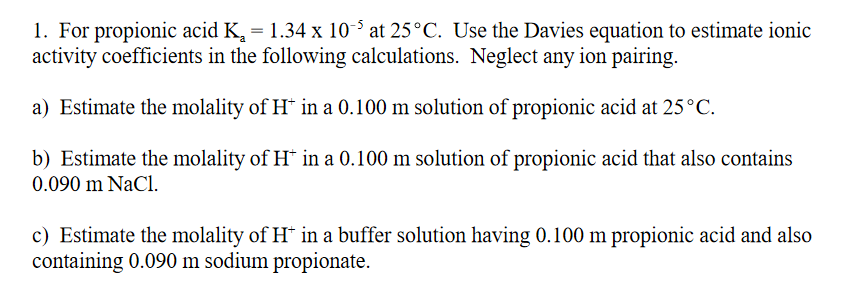
1. For propionic acid Ka=1.34105 at 25C. Use the Davies equation to estimate ionic activity coefficients in the following calculations. Neglect any ion pairing. a) Estimate the molality of H+in a 0.100m solution of propionic acid at 25C. b) Estimate the molality of H+in a 0.100m solution of propionic acid that also contains 0.090mNaCl. c) Estimate the molality of H+in a buffer solution having 0.100m propionic acid and also containing 0.090m sodium propionate
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


