Question: THIS IS A THREE PART QUESTION. THIS IS PART 2 A triester that contains three identical R groups can be extracted from coconut oil. This
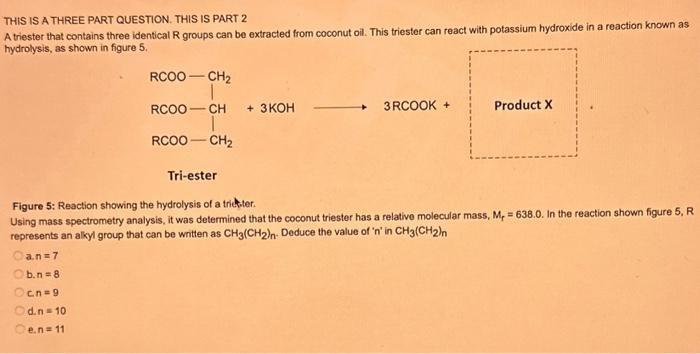
THIS IS A THREE PART QUESTION. THIS IS PART 2 A triester that contains three identical R groups can be extracted from coconut oil. This triester can react with potassium hydroxide in a reaction known as hydrolysis, as shown in figure 5. +3KOH3RCOOK+ProductX Tri-ester Figure 5: Reaction showing the hydrolysis of a trittter. Using mass spectrometry analysis, it was determined that the coconut triester has a relative molecular mass, Mr=638.0. In the reaction shown figure 5 , R represents an alkyl group that can be wnitten as CH3(CH2)n. Deduce the value of ' n ' in CH3(CH2)n a. n=7 b. n=8 c n=9 d.n=10 e.n=11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


