Question: THIS IS A THREE PART QUESTION. THIS IS PART 3 A triester that contains three identical R groups can be extracted from coconut oil. This
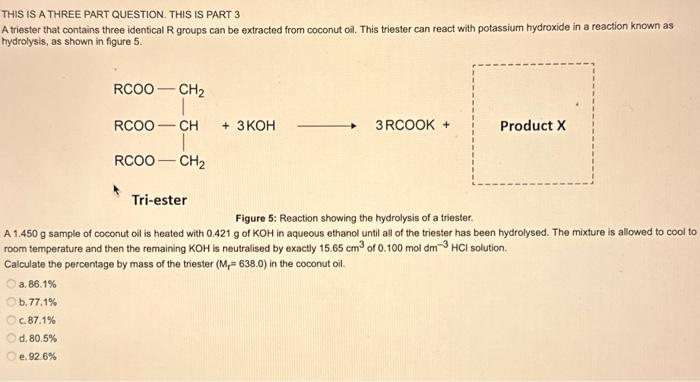
THIS IS A THREE PART QUESTION. THIS IS PART 3 A triester that contains three identical R groups can be extracted from coconut oil. This triester can react with potassium hydroxide in a reaction known as hydrolysis, as shown in figure 5 . +3KOH3RCOOK+ProductX Tri-ester Figure 5: Reaction showing the hydrolysis of a triester. A 1.450g sample of coconut oil is heated with 0.421g of KOH in aqueous ethanol until all of the triester has been hydrolysed. The mixture is allowed to cool to room temperature and then the remaining KOH is neutralised by exactly 15.65cm3 of 0.100moldm3HCl solution. Calculate the percentage by mass of the triester (Mr=638.0) in the coconut oil. a. 86.1% b. 77.1% c. 87.1% d. 80.5% e. 92.6%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


