Question: this is all a one question please with different parts can you help me? thank you!! Sulfuric acid is used in the synthesis and processing
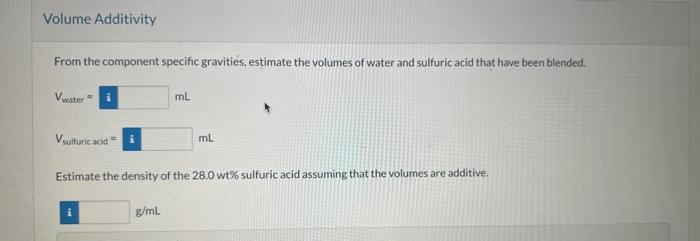
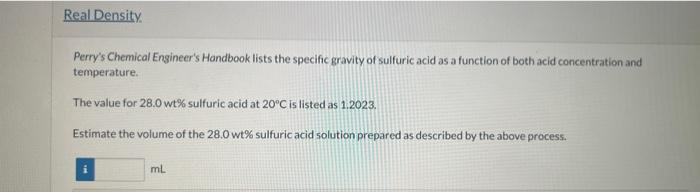
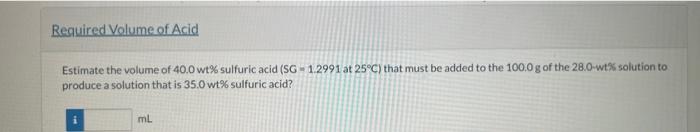
Sulfuric acid is used in the synthesis and processing of many chemicals and metals, and it is the electrolyte in common lead-acid batteries It is used in various strengths, ranging from concentrated (100\%) to dilute; so being able to estimate its concentration from a simple measurement of specific gravity is quite useful. gravity of the solution to that availab from the literature To 72.0g of water you carefully add 28.0g of H2SO4 and allow the resulting mixture to equilibrate at 20C. (You know that rapidly adding the acid to water or water to concentrated acid can both lead to dangerous splatters of liquid.) Physical Property Tables From the component specific gravities, estimate the volumes of water and sulfuric acid that have been blended. Vwater=Vsulfuricacid=mL Estimate the density of the 28.0 wt\% sulfuric acid assuming that the volumes are additive. g/mL Perry's Chemical Engineer's Hondbook lists the specific gravity of sulfuric acid as a function of both acid concentration and temperature. The value for 28.0 wt\% sulfuric acid at 20C is listed as 1:2023. Estimate the volume of the 28.0 wt\% sulfuric acid solution prepared as described by the above process. Estimate the volume of 40.0 wt % sulfuric acid (SG =1.2991 at 25C ) that must be added to the 100.0g of the 28.0 -wt 5 solution to produce a solution that is 35.0wt% sulfuric acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


