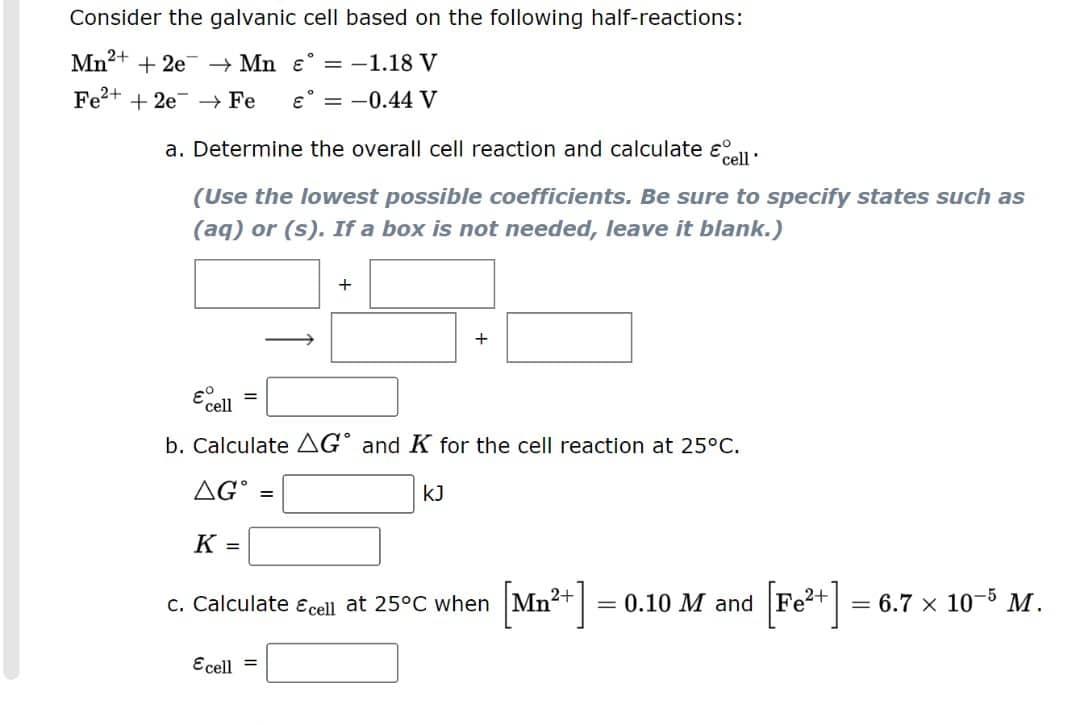
Question: This is my last attempt please help me Consider the galvanic cell based on the following half-reactions: 2+ Mn+ + 2e Mn = -1.18 V

This is my last attempt please help me
Consider the galvanic cell based on the following half-reactions: 2+ Mn+ + 2e Mn = -1.18 V Fe+ + 2e Fe = -0.44 V a. Determine the overall cell reaction and calculate cell' (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) + + = cell b. Calculate AG and K for the cell reaction at 25C. G = K = c. Calculate & cell at 25C when [Mn+] = 0.10 M and [Fe+] = 6.7 x 10-5 M. Ecell = RY [References] INTERACTIVE EXAMPLE Electroplating How long will it take to plate out 3.0 g Ni from aqueous Ni2+ with a current of 108.4 A? HOW DO WE GET THERE? What is the quantity of charge carried by these electrons (in C)? X C Recheck Next (4 of 5) 7th attempt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


