Question: This is not a Stoke regime solve according it is not similar to Part 1 Problem Statement Consider a very large container filled with water

This is not a Stoke regime solve according it is not similar to Part 1
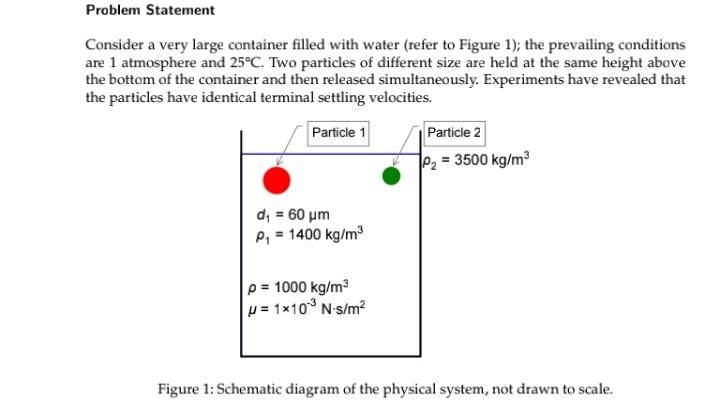
Problem Statement Consider a very large container filled with water (refer to Figure 1); the prevailing conditions are 1 atmosphere and 25C. Two particles of different size are held at the same height above the bottom of the container and then released simultaneously. Experiments have revealed that the particles have identical terminal settling velocities. Particle 1 Particle 2 122 = 3500 kg/m d. = 60 um P, = 1400 kg/m3 p = 1000 kg/m2 = 110Nis/m2 Figure 1: Schematic diagram of the physical system, not drawn to scale. Part 2 Now consider virtually the same problem as in Part 1, but this time water is replaced by 1- pentene. Once again, determine the height difference between the particles when each particle has attained 95% of its terminal settling velocity. Properties of 1-pentene: p = 650 kg/m3; J = 0.15 x 10-3 N-s/m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


