Question: this is our question and the help sheet we are suppose to go by Question 45 points) Solve this problem on your own paper. Do
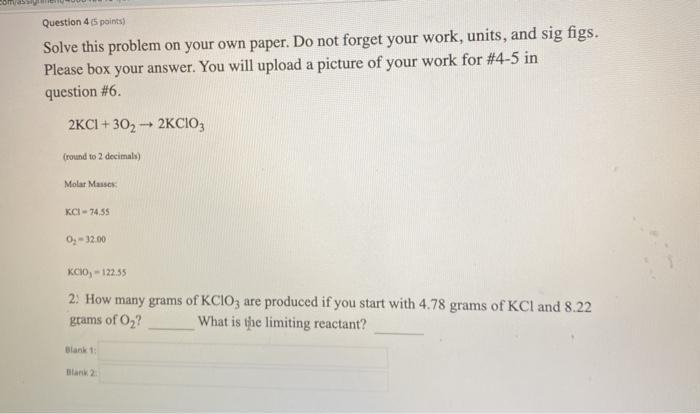
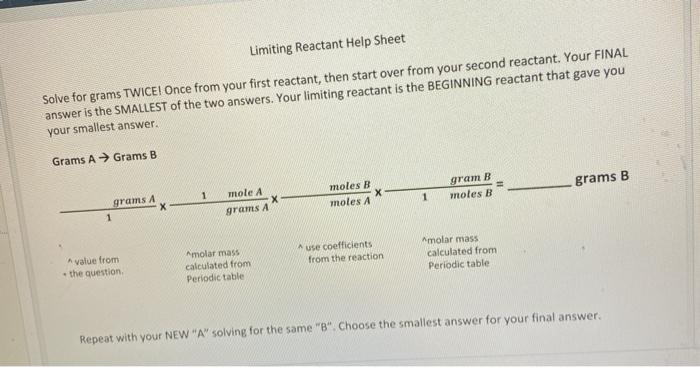
Question 45 points) Solve this problem on your own paper. Do not forget your work, units, and sig figs. Please box your answer. You will upload a picture of your work for #4-5 in question #6. 2KCI+ 302 - 2KCIO, (round to 2 decimals) Molar Masse KCI -74,55 O, -12.00 KCIO, - 122.35 2. How many grams of KCIO3 are produced if you start with 4.78 grams of KCl and 8.22 grams of Oz? What is the limiting reactant? Limiting Reactant Help Sheet Solve for grams TWICE! Once from your first reactant, then start over from your second reactant. Your FINAL answer is the SMALLEST of the two answers. Your limiting reactant is the BEGINNING reactant that gave you your smallest answer. Grams A Grams B gram B grams B 1 moles B moles 1 mole A grams A moles B grams A X 1 use coefficients from the reaction molar mass calculated from Periodic table molar mass calculated from Periodic table A value from the question Repeat with your NEW "A" solving for the same "B" Choose the smallest answer for your final
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


