Question: this is the question.do it correctly showing all possible and required steps plus main focus is of graph so plot on a neat paper making
 this is the question.do it correctly showing all possible and required steps plus main focus is of graph so plot on a neat paper making it easier to understand.Please do correct work i will rate your work and my friends will give like as well thanks.Try to deliver it by today
this is the question.do it correctly showing all possible and required steps plus main focus is of graph so plot on a neat paper making it easier to understand.Please do correct work i will rate your work and my friends will give like as well thanks.Try to deliver it by today
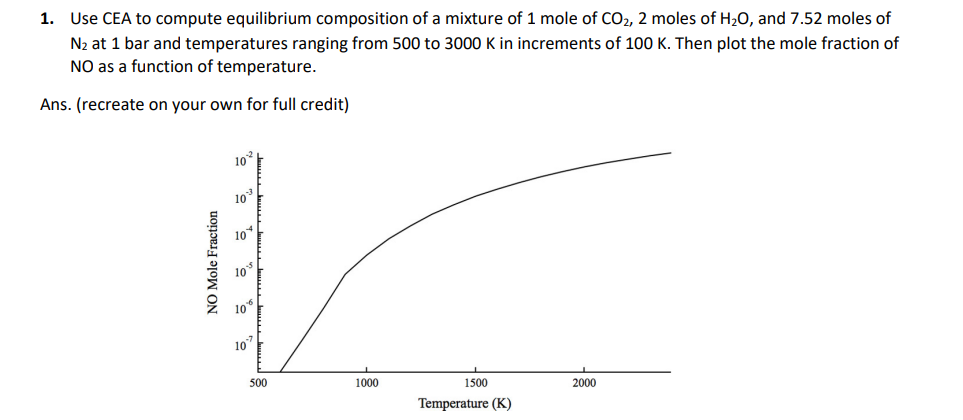
1. Use CEA to compute equilibrium composition of a mixture of 1 mole of CO2, 2 moles of H20, and 7.52 moles of N2 at 1 bar and temperatures ranging from 500 to 3000 K in increments of 100 K. Then plot the mole fraction of NO as a function of temperature. Ans. (recreate on your own for full credit) 10 10 10 NO Mole Fraction 10 lo 10 500 1000 1500 2000 Temperature (K) 1. Use CEA to compute equilibrium composition of a mixture of 1 mole of CO2, 2 moles of H20, and 7.52 moles of N2 at 1 bar and temperatures ranging from 500 to 3000 K in increments of 100 K. Then plot the mole fraction of NO as a function of temperature. Ans. (recreate on your own for full credit) 10 10 10 NO Mole Fraction 10 lo 10 500 1000 1500 2000 Temperature (K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


