Question: This is the work for a prior question. Please answer the question at the bottom. With assumptions: If the soda were cooled to 5C would
This is the work for a prior question. Please answer the question at the bottom. With assumptions:
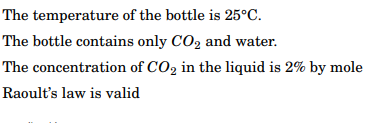


If the soda were cooled to 5C would you get a better fizz? Perform enough calculations to validate your answer and indicate any ap- proximations you needed to make.
The temperature of the bottle is 25C. The bottle contains only CO2 and water. The concentration of CO2 in the liquid is 2% by mole Raoult's law is valid Solution in bottle OF the pessure Now, calculating is OF CO- Soda mole have 2% by - 100ml bottle Water soda OF CO and total moles let CO, OF & % OF 100mol amoc moles 0 OF moles - 100 - 98 moc 250 bottle - OF 25 +23-15 temperature 98.15% bottle is IL of coolume assume Let us that , know we Here, R = Gas PV=nRT P- HRT NRT/v constant - 0.08206 Latm mock-! po 2 mol x 0.08206 X298.15 IL = CO2 OF poessure OF CO2 48.93 atm numbey OF moles Of Mol OF CO2 number Fraction total mole amol 100 moc - 0.02 Foom Raoult's law, Of Solcrtion = PxA pressure = Peoa 2002 # 48.93 atm x 0.02 or bottle poessure -0.9786 atm = 0.98 atm. 10.98 atm bottle Sada OF pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


