Question: This is using MATLAB, please explain as much as you can so i can understand the coding and learn from it! Thank you Ammonia (NH3)
This is using MATLAB, please explain as much as you can so i can understand the coding and learn from it! Thank you
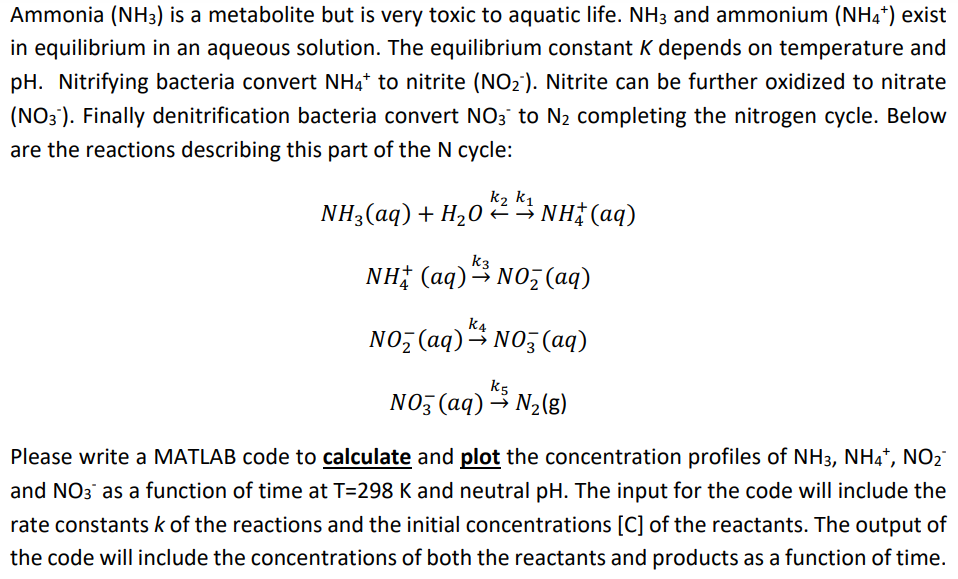
Ammonia (NH3) is a metabolite but is very toxic to aquatic life. NH3 and ammonium (NH4+) exist in equilibrium in an aqueous solution. The equilibrium constant K depends on temperature and pH. Nitrifying bacteria convert NH4 to nitrite (NO2'). Nitrite can be further oxidized to nitrate (NO3'). Finally denitrification bacteria convert NO3 to N2 completing the nitrogen cycle. Below are the reactions describing this part of the N cycle: NH3(aq) + H2O + NH(aq) 03 * ! k3 NH(aq) = N02 (aq) k4 Noz (aq)"- NOz (aq) kg N03(aq) N2(g) Please write a MATLAB code to calculate and plot the concentration profiles of NH3, NH4+, NO2 and NO3' as a function of time at T=298 K and neutral pH. The input for the code will include the rate constants k of the reactions and the initial concentrations (C) of the reactants. The output of the code will include the concentrations of both the reactants and products as a function of time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


