Question: This is using MATLAB, please explain as much as you can so I can understand the coding and learn from it! Thank you K1 NH3(aq)
This is using MATLAB, please explain as much as you can so I can understand the coding and learn from it! Thank you
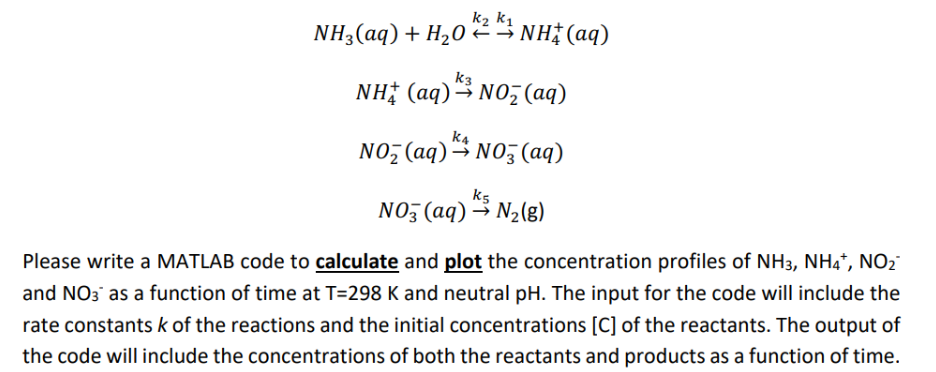
K1 NH3(aq) + H02 NH(aq) k3 NH+ (aq) NO (aq) K4 NO (aq) NO3(aq) k5 NO3(aq) N(g) Please write a MATLAB code to calculate and plot the concentration profiles of NH3, NH4+, NO and NO3 as a function of time at T=298 K and neutral pH. The input for the code will include the rate constants k of the reactions and the initial concentrations [C] of the reactants. The output of the code will include the concentrations of both the reactants and products as a function of time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


