Question: This problem is ment to be solved in the MATLAB program. I will give thumbs up for answers! thanks for any and all help! My
This problem is ment to be solved in the MATLAB program. I will give thumbs up for answers! thanks for any and all help!
My solution to problem 2 as stated in the problem above is as follows:
function[a b]=myline(x,y) n=1; a=(sum(x)*sum(y)-(n*sum(x.*y)))/((sum(x).^2)-(n*sum(x.^2))); b=(sum(x)*sum(x.*y)-sum(x.^2)*sum(y))/((sum(x).^2)-(n*sum(x.^2))); end
T = [200 400 600 800 1000];
k = [1.46*10^-7 0.0012 0.0244 0.1099 0.2710];
R = 8.314;
lnk = log(k);
OneByT = 1./T;
plot(OneByT,lnk); % Part a
xlabel('1/T');ylabel('ln(k)');
[mQByR,lnko] = myline(OneByT,lnk);% Part b
fprintf('-Q/R = %f ln(ko) = %f ',mQByR,lnko);
T = 500; % Part c
k = lnko + mQByR/T;
fprintf('k = %f for T = 500K ',k);
Q = -mQByR*R; % Part d
fprintf('Q = %f ',Q);
ko = exp(lnko); % Part e
fprintf('ko = %f ',ko);
Output:
-Q/R = -2487.661658 ln(ko) = -1.291282 k = -6.266605 for T = 500K Q = 20682.419024 ko = 0.274918
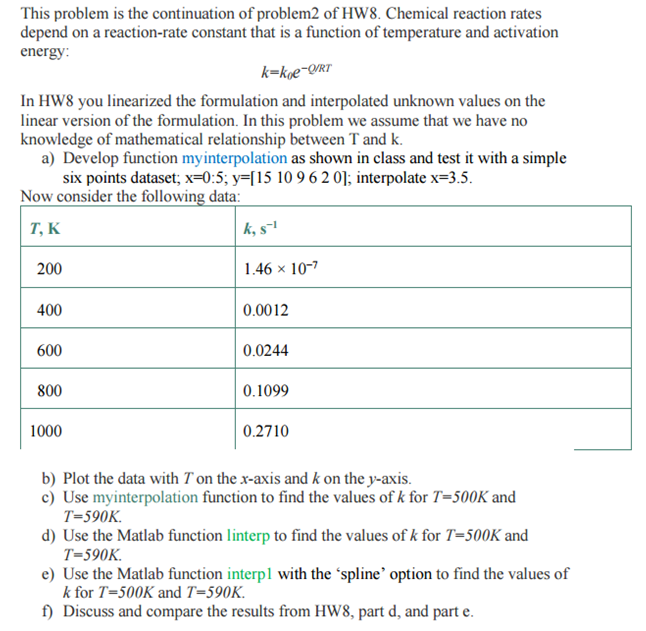
This problem is the continuation of problem2 of HW8. Chemical reaction rates depend on a reaction-rate constant that is a function of temperature and activation energy k koe-on In HW8 you linearized the formulation and interpolated unknown values on the linear version of the formulation. In this problem we assume that we have no knowledge of mathematical relationship between Tand k. a) Develop function myinterpolation as shown in class and test it with a simple six points dataset, x 0:5, y l 15 10 96 2 01, interpolate x 3.5. Now consider the following data: k, s T, K 1.46 x 10-7 200 400 0.0012 600 0.0244 800 0.1099 0.2710 1000 b) Plot the data with Ton the x-axis and k on the y-axis. c) Use my interpolation function to find the values of k for T 500K and T 590K. d) Use the Matlab function linterp to find the values of k for T 500K and T 590K. e) Use the Matlab function interpl with the spline' option to find the values of k for T 500K and T 590K. f Discuss and compare the results from HW8, part d, and part e. This problem is the continuation of problem2 of HW8. Chemical reaction rates depend on a reaction-rate constant that is a function of temperature and activation energy k koe-on In HW8 you linearized the formulation and interpolated unknown values on the linear version of the formulation. In this problem we assume that we have no knowledge of mathematical relationship between Tand k. a) Develop function myinterpolation as shown in class and test it with a simple six points dataset, x 0:5, y l 15 10 96 2 01, interpolate x 3.5. Now consider the following data: k, s T, K 1.46 x 10-7 200 400 0.0012 600 0.0244 800 0.1099 0.2710 1000 b) Plot the data with Ton the x-axis and k on the y-axis. c) Use my interpolation function to find the values of k for T 500K and T 590K. d) Use the Matlab function linterp to find the values of k for T 500K and T 590K. e) Use the Matlab function interpl with the spline' option to find the values of k for T 500K and T 590K. f Discuss and compare the results from HW8, part d, and part e
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


