Question: This problem is talking about equilibrium and Le Chatelier's Principle but I am so lost on what it's asking i've gotten as fat as putting
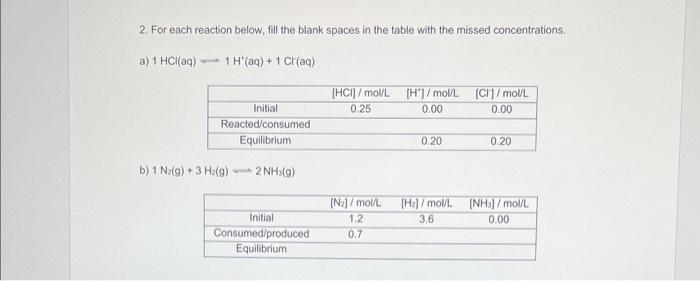
2. For each reaction below, fill the blank spaces in the table with the missed concentrations. a) 1HCl(aq)=1H+(aq)+1Cl(aq) b) 1N2(g)+3H2(g)2NH3(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


