Question: This question does exist on Chegg, but it doesn't actually answer the question. Any help is appreciated! 2. It has been proposed that a suitable
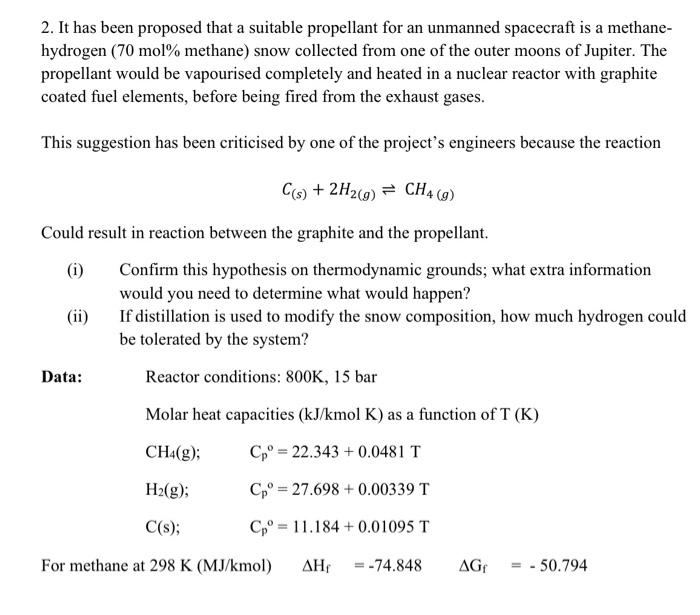
2. It has been proposed that a suitable propellant for an unmanned spacecraft is a methanehydrogen ( 70mol% methane) snow collected from one of the outer moons of Jupiter. The propellant would be vapourised completely and heated in a nuclear reactor with graphite coated fuel elements, before being fired from the exhaust gases. This suggestion has been criticised by one of the project's engineers because the reaction C(s)+2H2(g)CH4(g) Could result in reaction between the graphite and the propellant. (i) Confirm this hypothesis on thermodynamic grounds; what extra information would you need to determine what would happen? (ii) If distillation is used to modify the snow composition, how much hydrogen could be tolerated by the system? Data: Reactor conditions: 800K,15 bar Molar heat capacities (kJ/kmolK) as a function of T(K) CH4(g);H2(g);C(s);Cp=22.343+0.0481TCpo=27.698+0.00339TCp0=11.184+0.01095T For methane at 298K(MJ/kmol)Hf=74.848Gf=50.794
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


