Question: This question has multiple parts. Work all the parts to get the most points. Balance each of the following chemical equations. (Use the lowest
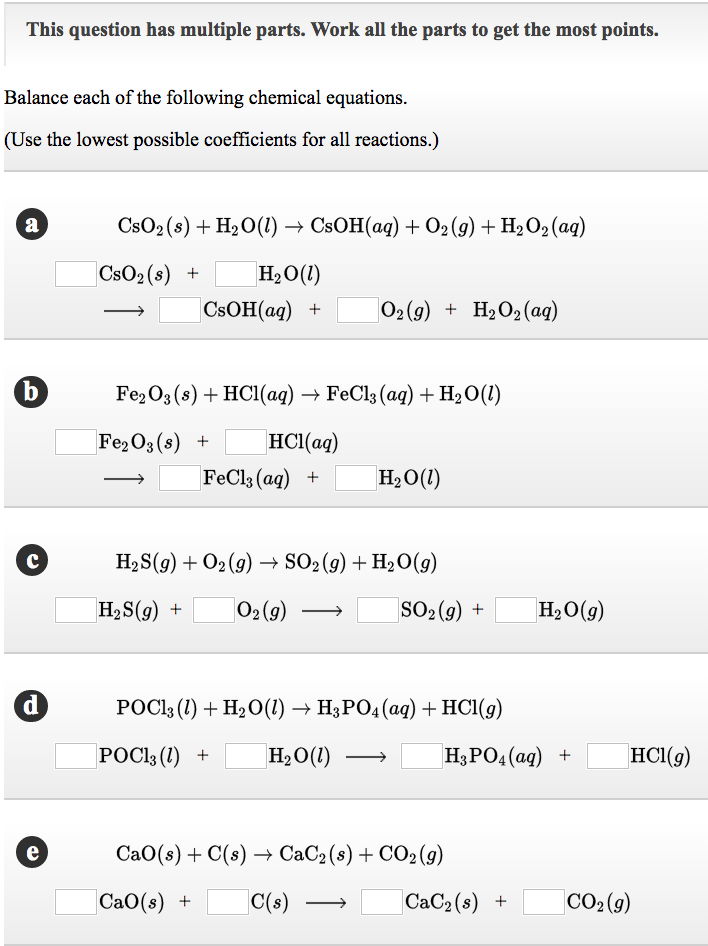
This question has multiple parts. Work all the parts to get the most points. Balance each of the following chemical equations. (Use the lowest possible coefficients for all reactions.) a b d e CsO2 (s) + HO(l) CsOH(aq) + O2(g) + HO (aq) CsO (s) + HO(l) CSOH(aq) + O2(g) + HO (aq) Fe2O3 (s) + HCl(aq) FeCl3 (aq) + HO(1) FeO3(s) + HCl(aq) FeCl3(aq) + - HO(l) HS(g) + O(g) SO(g) + HO(g) HS(g) + O2(g) SO(g) + POC13 (1) + HO(1) H3PO4 (aq) + HCl(g) POC13 (1) + HO(1) H3PO4 (aq) + CaO(s) + C(s) CaC2 (s) + CO2(g) CaO(s) + C(s) HO(g) CaC (s) + HCl(g) CO(g)
Step by Step Solution
3.49 Rating (166 Votes )
There are 3 Steps involved in it
In order to balance the equation Stoichiometric coef... View full answer

Get step-by-step solutions from verified subject matter experts


