Question: This question is very clear. The Beaker shown is just a go by it is not the problem. The problem is the typed texted. It
This question is very clear. The Beaker shown is just a go by it is not the problem. The problem is the typed texted.
It is just to show you the image and we are using the general mass transfer equation.
Need help solving this mass transfer problem.
Thank you 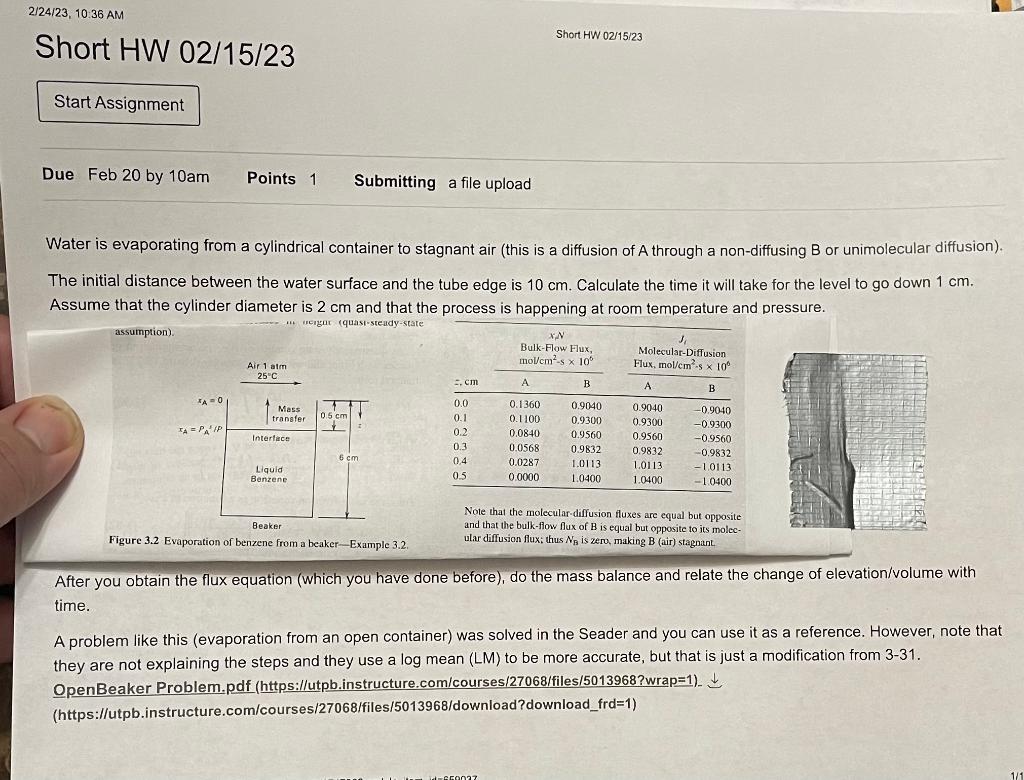
Water is evaporating from a cylindrical container to stagnant air (this is a diffusion of A through a non-diffusing B or unimolecular diffusion). The initial distance between the water surface and the tube edge is 10cm. Calculate the time it will take for the level to go down 1cm. Assume that the cylinder diameter is 2cm and that the process is happening at room temperature and pressure. assumption). Note that the molecular-diffusion fluxes are equal but opposite and that the bulk-flow flux of B is equal but opposite to its molec- Figure 3.2 Evaporation of benzene from a beaker-Example 3.2. ular diffusion flux; thus NB is zero, making B (air) stagnant After you obtain the flux equation (which you have done before), do the mass balance and relate the change of elevation/volume with time. A problem like this (evaporation from an open container) was solved in the Seader and you can use it as a reference. However, note that they are not explaining the steps and they use a log mean (LM) to be more accurate, but that is just a modification from 3-31. OpenBeaker Problem.pdf (https://utpb.instructure.com/courses/27068/files/5013968?wrap=1) (https://utpb.instructure.com/courses/27068/files/5013968/download?download_frd=1)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


