Question: this question please thank you ! question 2 here 3. A student forgot to measure the temperature of the water bath for the gas in

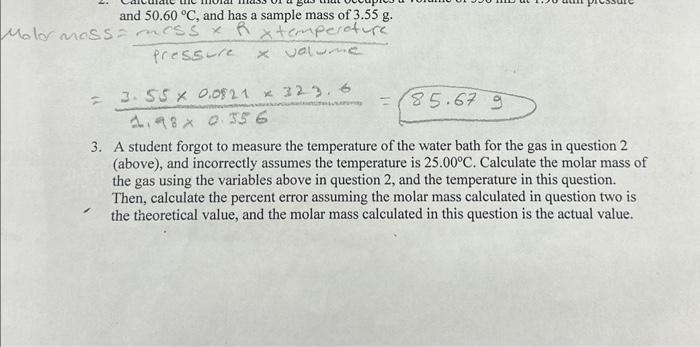
3. A student forgot to measure the temperature of the water bath for the gas in question 2 (above), and incorrectly assumes the temperature is 25.00C. Calculate the molar mass of the gas using the variables above in question 2 , and the temperature in this question. Then, calculate the percent error assuming the molar mass calculated in question two is the theoretical value, and the molar mass calculated in this question is the actual value. and 50.60C, and has a sample mass of 3.55g. SS=pressurexvalmencemosShtcmperoture=1.980.5563.550.082132.6.6=85.679 3. A student forgot to measure the temperature of the water bath for the gas in question 2 (above), and incorrectly assumes the temperature is 25.00C. Calculate the molar mass of the gas using the variables above in question 2, and the temperature in this question. Then, calculate the percent error assuming the molar mass calculated in question two is the theoretical value, and the molar mass calculated in this question is the actual value
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


