Question: This question requires a numerical answer. Type only the numerical value. Do NOT include units and do NOT use scientific notation. Give your answer to
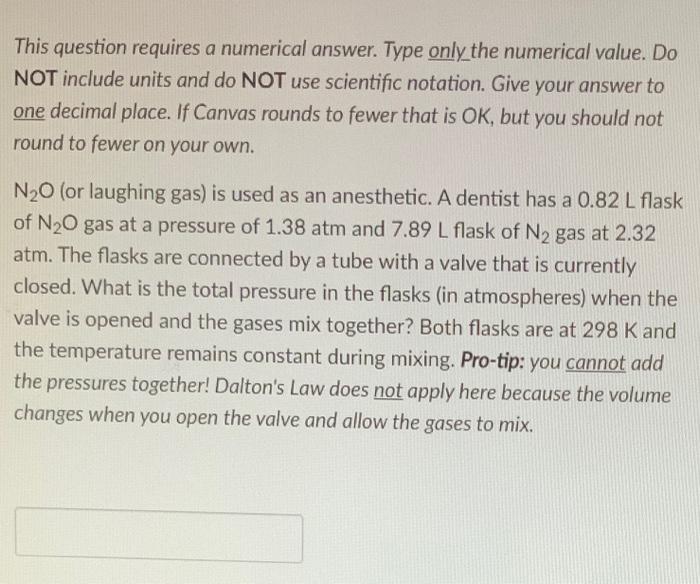
This question requires a numerical answer. Type only the numerical value. Do NOT include units and do NOT use scientific notation. Give your answer to one decimal place. If Canvas rounds to fewer that is OK, but you should not round to fewer on your own. N20 (or laughing gas) is used as an anesthetic. A dentist has a 0.82 L flask of N20 gas at a pressure of 1.38 atm and 7.89 L flask of N2 gas at 2.32 atm. The flasks are connected by a tube with a valve that is currently closed. What is the total pressure in the flasks (in atmospheres) when the valve is opened and the gases mix together? Both flasks are at 298 K and the temperature remains constant during mixing. Pro-tip: you cannot add the pressures together! Dalton's Law does not apply here because the volume changes when you open the valve and allow the gases to mix
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


