Question: Three flasks, each containing a gas sample, are set up as shown below. Assuming no temperature change, determine the final pressure inside the system after
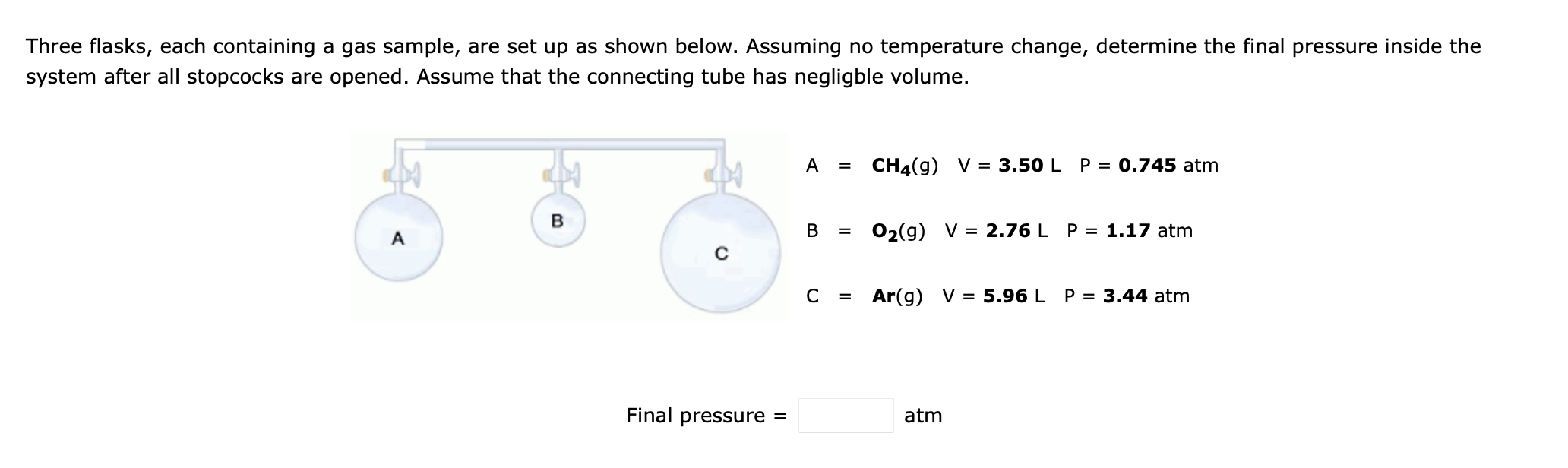
Three flasks, each containing a gas sample, are set up as shown below. Assuming no temperature change, determine the final pressure inside the system after all stopcocks are opened. Assume that the connecting tube has negligble volume. A = CH4(g) V = 3.50 L P = 0.745 atm B B A O2(g) V = 2.76 L P = 1.17 atm C = Ar(g) V = 5.96 L P = 3.44 atm Final pressure = atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


