Question: Three separate solutions are described below. Each substance is dissolved in water. Calculate the missing values. density of solution percent by massmolarity Solution molality Id
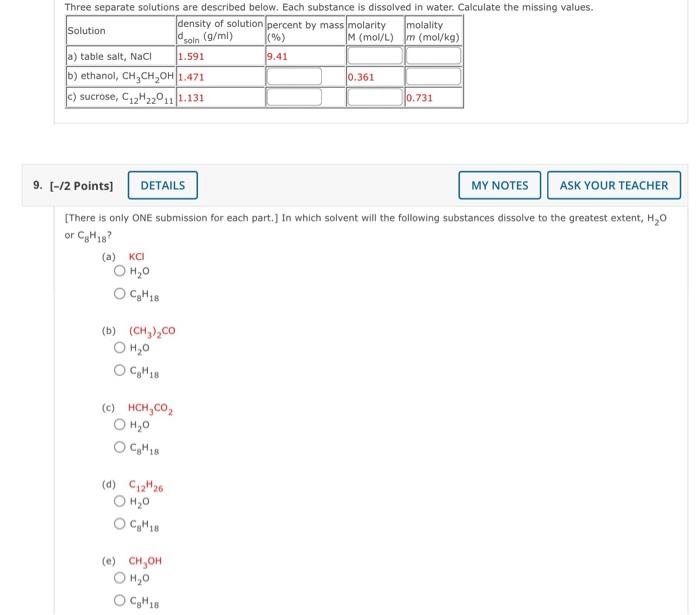
Three separate solutions are described below. Each substance is dissolved in water. Calculate the missing values. density of solution percent by massmolarity Solution molality Id soin (g/ml) (%) M (mol/L) m (mol/kg) a) table salt, Naci 1.591 9.41 b) ethanol, CH,CH,OH 1.471 0.361 c) sucrose, C12H22011 1.131 0.731 9. (-/2 points) DETAILS MY NOTES ASK YOUR TEACHER [There is only ONE submission for each part.] In which solvent will the following substances dissolve to the greatest extent, H,0 or CH28? (a) KCI OH,O 1 Cg H18 (b) (CH3),CO OH,0 OC,M18 (c) HCH,CO2 OH,O OCM18 (d) C2M26 ,9 OCM18 (e) CH,OH OH,0 OCH 18
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


