Question: thx! 5. (15 points) See the data table below for two volatile substances. For an ideal solution containing 750.0mL of ethanol and 250.0mL of toluene,
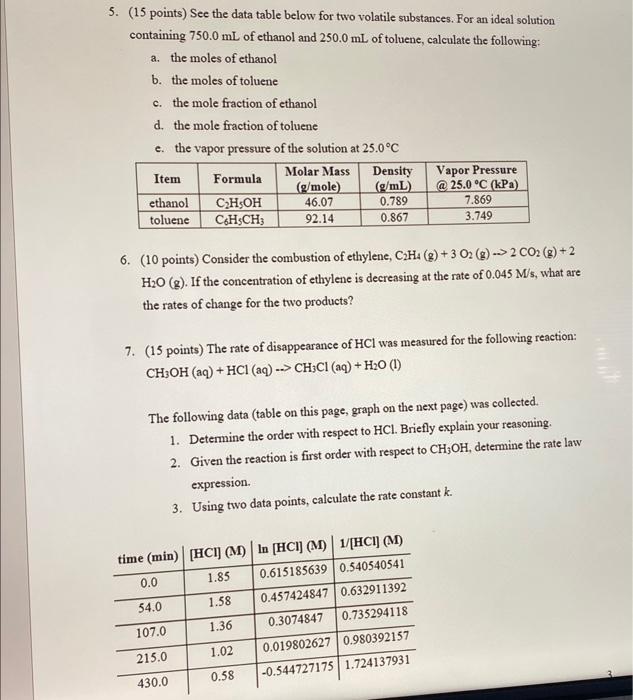
5. (15 points) See the data table below for two volatile substances. For an ideal solution containing 750.0mL of ethanol and 250.0mL of toluene, calculate the following: a. the moles of ethanol b. the moles of toluene c. the mole fraction of ethanol d. the mole fraction of toluene e. the vapor pressure of the solution at 25.0C 6. (10 points) Consider the combustion of ethylene, C2H4(g)+3O2(g)2CO2(g)+2 H2O(g). If the concentration of ethylene is decreasing at the rate of 0.045M/s, what are the rates of change for the two products? 7. (15 points) The rate of disappearance of HCl was measured for the following reaction: CH3OH(aq)+HCl(aq)CH3Cl(aq)+H2O(l) The following data (table on this page, graph on the next page) was collected. 1. Determine the order with respect to HCl. Briefly explain your reasoning. 2. Given the reaction is first order with respect to CH3OH, determine the rate law expression. 3. Using two data points, calculate the rate constant k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


