Question: Explain which of these reaction proceeds at a faster rate: CH3 a) CHC-Cl + CHCOH CH Br b) CHCHCHCH CH3O Br c) CHCH,CH,CH, + CHO
Explain which of these reaction proceeds at a faster rate:
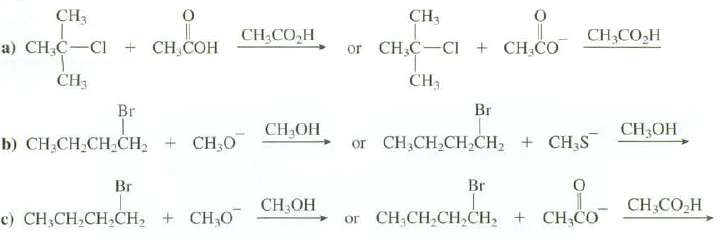
CH3 a) CHC-Cl + CHCOH CH Br b) CHCHCHCH CH3O Br c) CHCH,CH,CH, + CHO CH CO,H CHOH CHOH CH3 O or CHC CI+ CHCO CH3 Br CH3COH or CHCHCHCH + CH3S Br or CHCHCHCHCHCO 0 CHOH CH,CO_H
Step by Step Solution
3.53 Rating (167 Votes )
There are 3 Steps involved in it
a Because the leaving group is on a tertiary carbon the reaction proceeds by an ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-N-R (11).docx
120 KBs Word File


