Question: Time (min) CBZ (UM) 0.5 5 Problem #2. Ferrate, Fe(VI), is an emerging water treatment chemical used to oxidize aquatic micropollutants and disinfect pathogenic microorganisms.
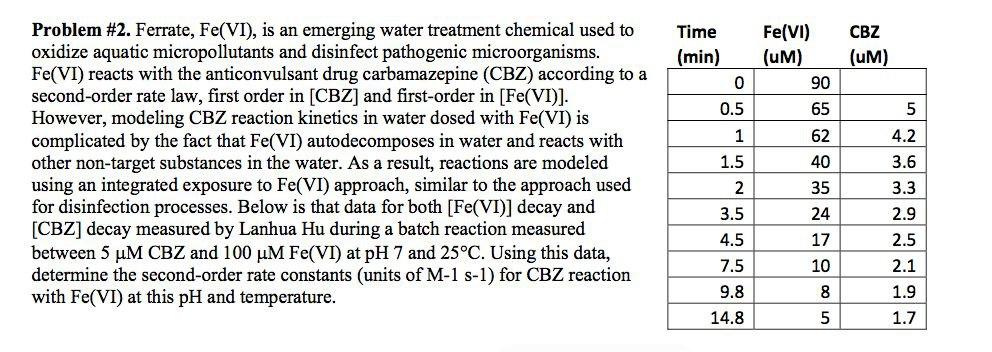
Time (min) CBZ (UM) 0.5 5 Problem #2. Ferrate, Fe(VI), is an emerging water treatment chemical used to oxidize aquatic micropollutants and disinfect pathogenic microorganisms. Fe(VI) reacts with the anticonvulsant drug carbamazepine (CBZ) according to a second-order rate law, first order in [CBZ] and first-order in [Fe(VI)]. However, modeling CBZ reaction kinetics in water dosed with Fe(VI) is complicated by the fact that Fe(VI) autodecomposes in water and reacts with other non-target substances in the water. As a result, reactions are modeled using an integrated exposure to Fe(VI) approach, similar to the approach used for disinfection processes. Below is that data for both [Fe(VI)] decay and [CBZ] decay measured by Lanhua Hu during a batch reaction measured between 5 uM CBZ and 100 uM Fe(VI) at pH 7 and 25C. Using this data, determine the second-order rate constants (units of M-1 S-1) for CBZ reaction with Fe(VI) at this pH and temperature. 1 1.5 oooo in in inno Fe(VI) (UM) 90 65 62 40 35 24 17 10 3 Un do EOS 8 3.5 4.5 4.2 3.6 3.3 2.9 2.5 2.1 1.9 1.7 7.5 9.8 14.8 8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


