Question: Time (s) [Trial 1 Temperature (C) Trial 2 Temperature (c) 0 84.4 30 83.7 60 80.8 90 80.1 120 79.3 150 77.6 180 77 210
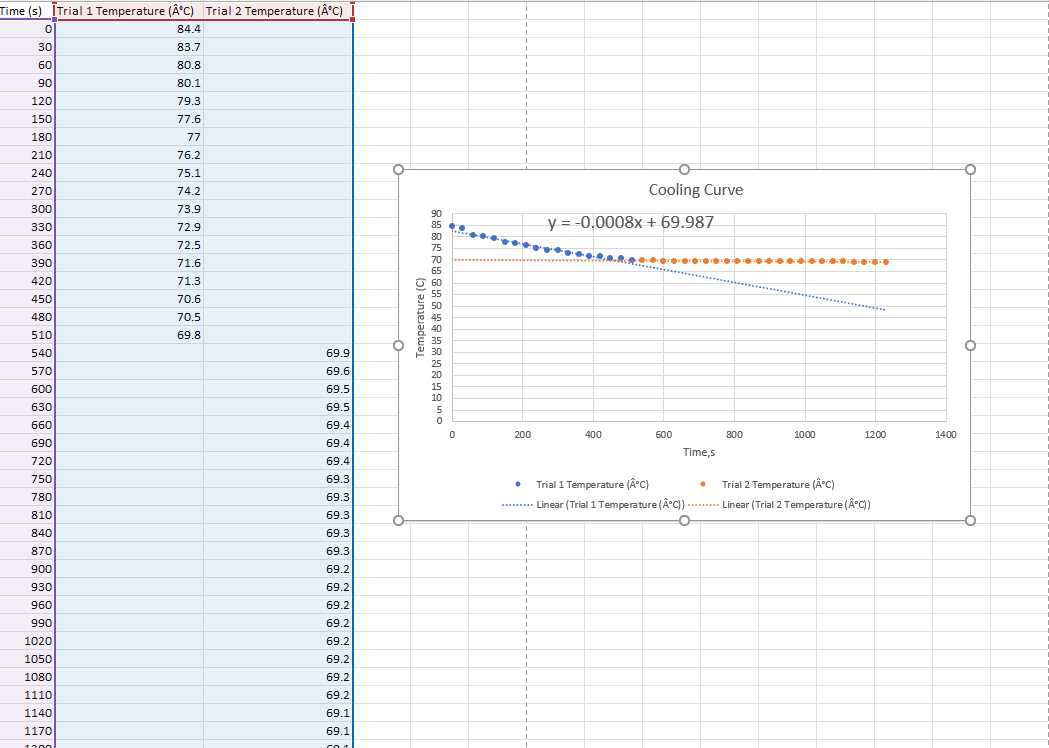
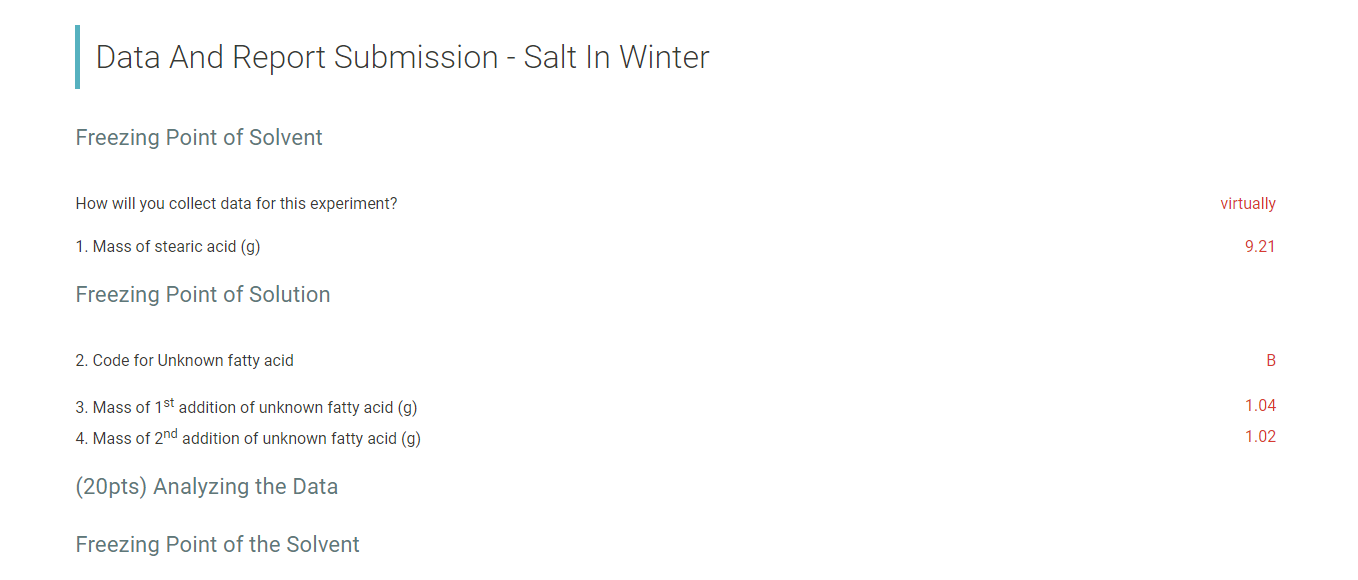

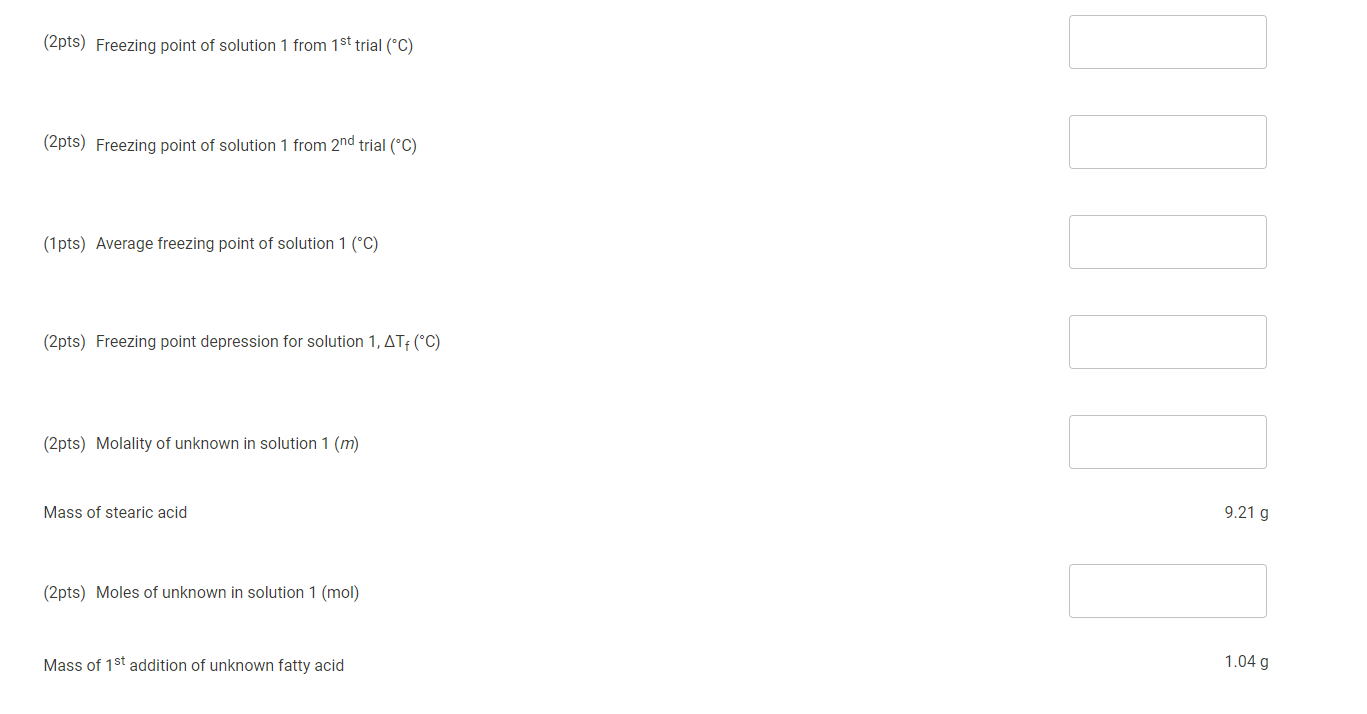
Time (s) [Trial 1 Temperature (C) Trial 2 Temperature (c) 0 84.4 30 83.7 60 80.8 90 80.1 120 79.3 150 77.6 180 77 210 76.2 240 75.1 270 74.2 Cooling Curve 300 73.9 72.9 72.5 y = -0.0008x + 69.987 ... 330 360 390 420 450 480 71.6 71.3 70.6 70.5 69.8 Temperature (C) OVER & 88 84 8488808 510 70 65 60 55 50 45 40 35 30 25 20 15 10 540 69.9 570 600 630 660 69.6 69.5 69.5 69.4 69.4 69.4 0 200 400 600 800 1000 1200 1400 690 720 Times . . 750 780 810 840 870 69.3 69.3 69.3 Trial 1 Temperature (C) Trial 2 Temperature (Ac) ........ Linear (Trial 1 Temperature (C))......... Linear (Trial 2 Temperature (C) ( ) 900 930 69.3 69.3 69.2 69.2 69.2 69.2 69.2 69.2 960 990 1020 1050 1080 1110 1140 1170 69.2 69.2 69.1 69.1 110 Data And Report Submission - Salt In Winter Freezing Point of Solvent How will you collect data for this experiment? virtually 1. Mass of stearic acid (9) 9.21 Freezing Point of Solution 2. Code for Unknown fatty acid B 1.04 3. Mass of 1st addition of unknown fatty acid (9) 4. Mass of 2nd addition of unknown fatty acid (9) 1.02 (20pts) Analyzing the Data Freezing Point of the Solvent (2pts) Freezing point of stearic acid from 1st trial (C) Saved (2pts) Freezing point of stearic acid from 2nd trial (C) Saved (1 pts) Average freezing point of stearic acid (C) Saved (30pts) Freezing Point of the Solution 1 (2pts) Freezing point of solution 1 from 1st trial (C) (2pts) Freezing point of solution 1 from 2nd trial (C) (1pts) Average freezing point of solution 1 (C) (2pts) Freezing point depression for solution 1, ATF (C) (2pts) Molality of unknown in solution 1 (m) Mass of stearic acid 9.21 g (2pts) Moles of unknown in solution 1 (mol) Mass of 1st addition of unknown fatty acid 1.04 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


