Question: The equilibrium between dissolved CO, and carbonic acid can be represented by H+ + HCO, H,CO, CO2 H,0 Show that dt where K- [HJEHCO,
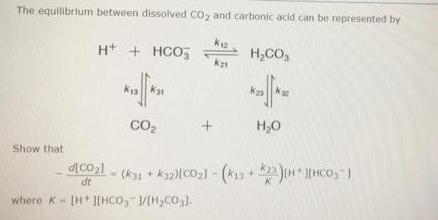
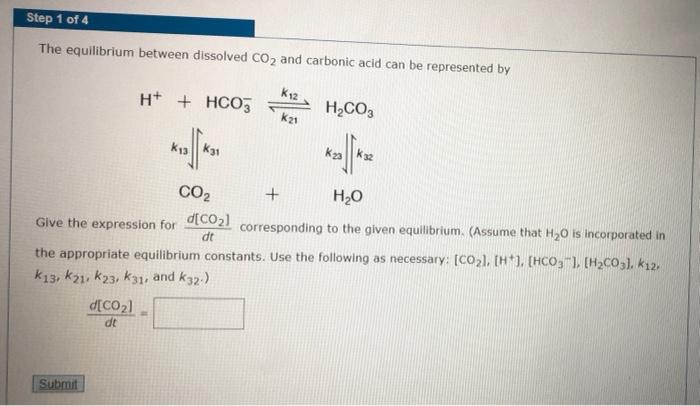
The equilibrium between dissolved CO, and carbonic acid can be represented by H+ + HCO, H,CO, CO2 H,0 Show that dt where K- [HJEHCO, /[H,CO,1. Step 1 of 4 The equilibrium between dissolved CO2 and carbonic acid can be represented by K12 H* + HCO5 H2CO3 k21 k31 CO2 H2O Give the expression for CO21 corresponding to the given equilibrium. (Assume that H,0 is incorporated in dt the appropriate equilibrium constants. Use the following as necessary: (CO2), (H*), [HCO,"), [H2CO3). k12. k13, k21, k23, kz1, and k32.) d[CO2) dt Submit
Step by Step Solution
3.38 Rating (164 Votes )
There are 3 Steps involved in it
Ht H0 Kal K23 H20 Co2 dcco dt KICCO d Clo2 kn Ht... View full answer

Get step-by-step solutions from verified subject matter experts


