Question: Titrations Workahoot For each problem, do the following: Write the balanced chemical equation. Use the information given to calculate the unknown Molarity. 1. In a
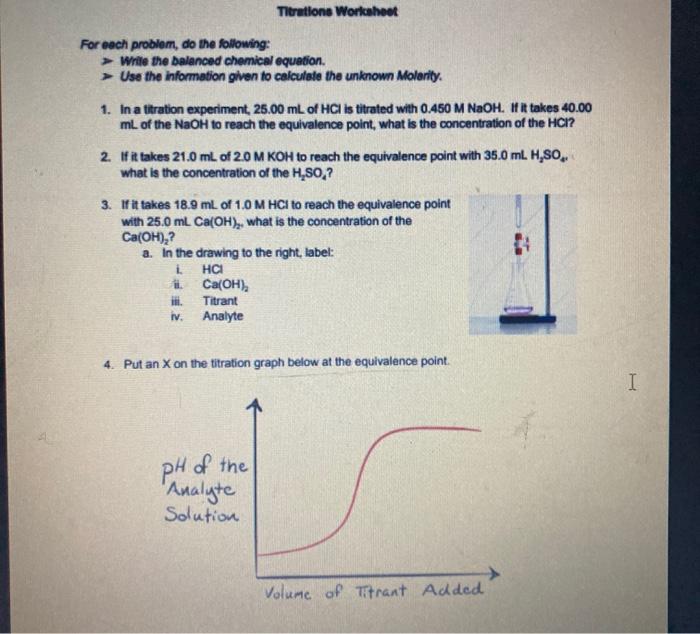
Titrations Workahoot For each problem, do the following: Write the balanced chemical equation. Use the information given to calculate the unknown Molarity. 1. In a titration experiment, 25.00 mL of HCI is titrated with 0.450 M NaOH. If it takes 40.00 ml of the NaOH to reach the equivalence point, what is the concentration of the HCI? 2. If it takes 21.0 mL of 2.0 M KOH to reach the equivalence point with 35.0 ml. H, what is the concentration of the H,SO,? 3. If it takes 18.9 mL of 1.0 M HCl to reach the equivalence point with 25.0 ml Ca(OH),, what is the concentration of the Ca(OH),? 3 a. In the drawing to the right, label: HCI Ca(OH), Titrant Analyte i IN IV. 4. Put an X on the titration graph below at the equivalence point. I I pH of the "Analyte Solution Volume of Titrant Added
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


