Question: TO CORRECT SIGNIFICANT FIGURES, PLEASE. Be sure to answer all parts. Calculate the vapor pressure of a solution made by dissolving 88.4g of urea (molar
TO CORRECT SIGNIFICANT FIGURES, PLEASE.
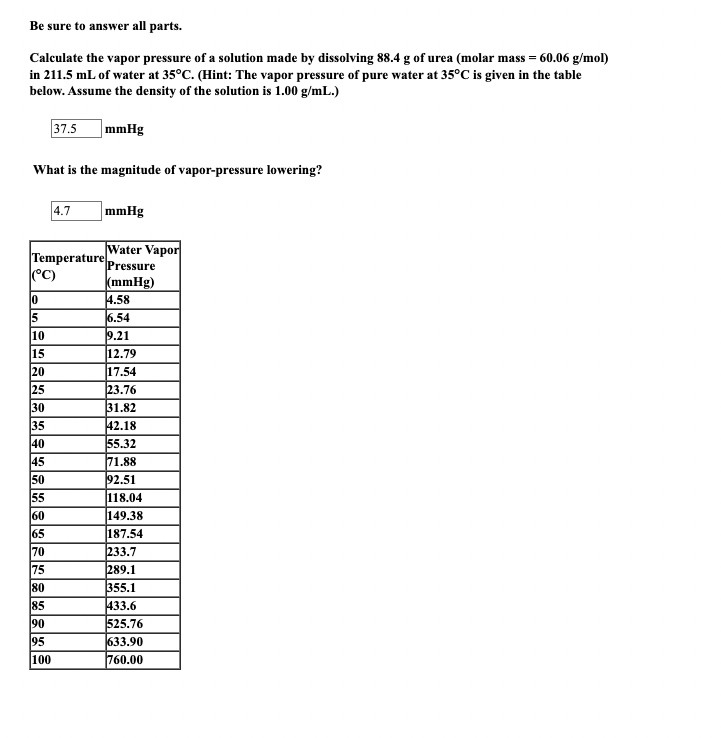
Be sure to answer all parts. Calculate the vapor pressure of a solution made by dissolving 88.4g of urea (molar mass =60.06g/mol ) in 211.5mL of water at 35C. (Hint: The vapor pressure of pure water at 35C is given in the table below. Assume the density of the solution is 1.00g/mL.) 37.5mmHg What is the magnitude of vapor-pressure lowering? 4.7mmHg \begin{tabular}{|l|l|} \hline Temperature (C) & Water Vapor Pressure (mmHg) \\ \hline 0 & 4.58 \\ \hline 5 & 6.54 \\ \hline 10 & 9.21 \\ \hline 15 & 12.79 \\ \hline 20 & 17.54 \\ \hline 25 & 23.76 \\ \hline 30 & 31.82 \\ \hline 35 & 42.18 \\ \hline 40 & 55.32 \\ \hline 45 & 71.88 \\ \hline 50 & 92.51 \\ \hline 55 & 118.04 \\ \hline 60 & 149.38 \\ \hline 65 & 187.54 \\ \hline 70 & 233.7 \\ \hline 75 & 289.1 \\ \hline 80 & 355.1 \\ \hline 85 & 433.6 \\ \hline 90 & 525.76 \\ \hline 95 & 633.90 \\ \hline 100 & 760.00 \\ \hline & \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


