Question: A freshly prepared sample of a certain radioactive isotope has an activity of 10.0 mCi. After 3.90 h, the activity is 7.80 mCi. (a)
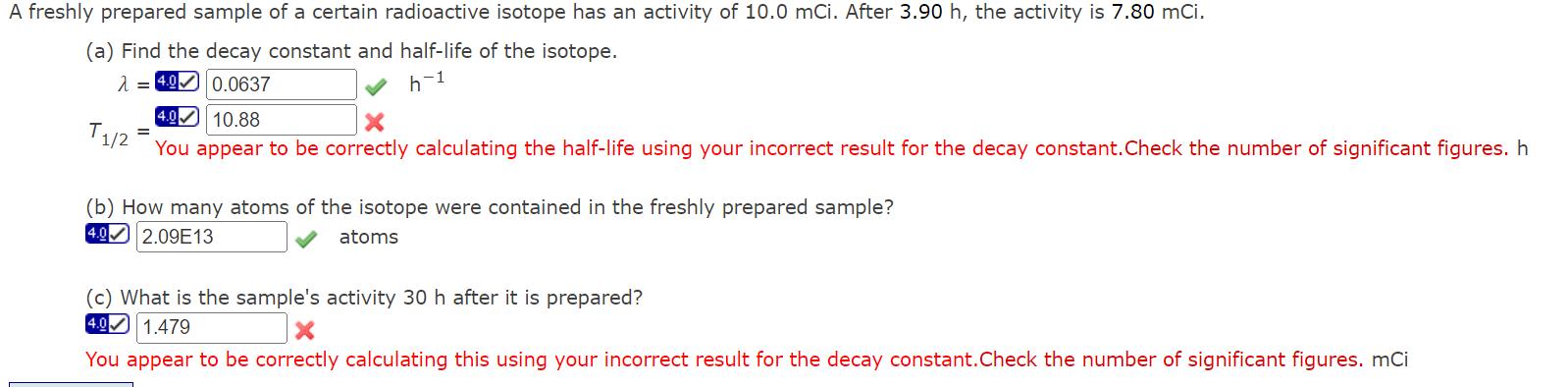
A freshly prepared sample of a certain radioactive isotope has an activity of 10.0 mCi. After 3.90 h, the activity is 7.80 mCi. (a) Find the decay constant and half-life of the isotope. T1/2 4.00.0637 4.0 10.88 h-1 You appear to be correctly calculating the half-life using your incorrect result for the decay constant. Check the number of significant figures. h (b) How many atoms of the isotope were contained in the freshly prepared sample? 4.02.09E13 atoms (c) What is the sample's activity 30 h after it is prepared? 4.01.479 You appear to be correctly calculating this using your incorrect result for the decay constant. Check the number of significant figures. mCi
Step by Step Solution
There are 3 Steps involved in it
Problem Summary A freshly prepared radioactive sample has Initial activity A 0 100 mCi A0 100 textmCi A0100mCi Activity after t 390 h t 390 texth t390... View full answer

Get step-by-step solutions from verified subject matter experts


