Question: Trial # Initial A (M) Initial B (M) Initial Rate (M/s) 0.200 0.200 1.20 x 10-5 12 0.200 0.400 2.40 x 10-5 0.400 0.200 4.80
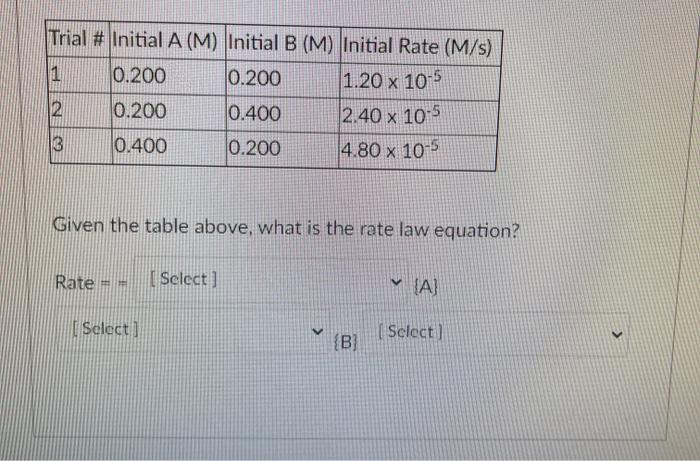
Trial # Initial A (M) Initial B (M) Initial Rate (M/s) 0.200 0.200 1.20 x 10-5 12 0.200 0.400 2.40 x 10-5 0.400 0.200 4.80 x 10-5 Given the table above, what is the rate law equation? Rate | Select] {A} I Select) V ( Select) > {B}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


