Question: Tutored Practice Probiem 15.4.1 Close Problem Predict and calculate the eflect of concentration changes on an equilibrium system. Some PCl5 is allowed to dissociate into
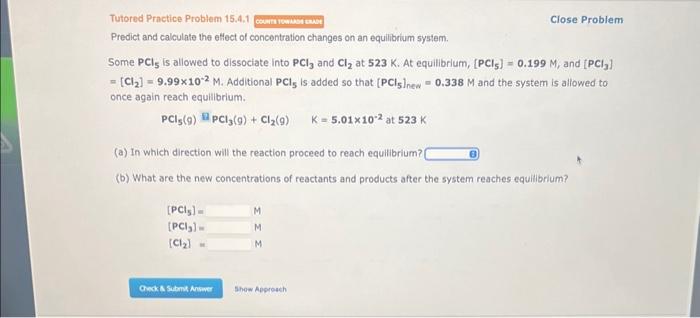
Tutored Practice Probiem 15.4.1 Close Problem Predict and calculate the eflect of concentration changes on an equilibrium system. Some PCl5 is allowed to dissociate into PCl3 and Cl2 at 523K.At equilibrium, [PCl5]=0.199M, and [PCl3] =[Cl2]=9.99102M. Additional PCl5 is added so that [PCl5]ne=0.338M and the system is allowed to once again reach equilibrium. PCl5(g)ZPCl3(9)+Cl2(g)K=5.01102at523K (a) In which direction will the reaction proceed to reach equilibrium? (b) What are the new concentrations of reactants and products after the system reaches equilibrium? [PCl5]=[PCl3]=[Cl2]=MMM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


