Question: Tutored Practice Problem 5.6.1 Close Problem Use and interpret standard heats of formation. (a) Write the balanced chemical equation that represents the standard heat of
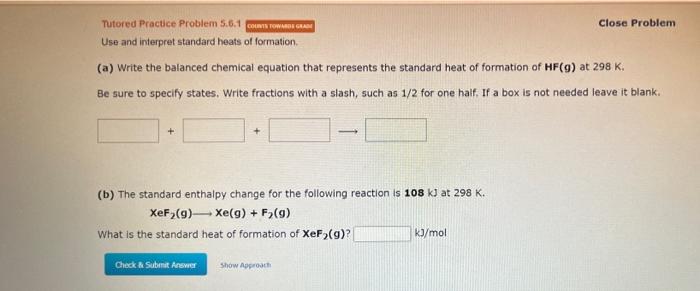
Tutored Practice Problem 5.6.1 Close Problem Use and interpret standard heats of formation. (a) Write the balanced chemical equation that represents the standard heat of formation of HF(g) at 298K. Be sure to specify states. Write fractions with a slash, such as 1/2 for one half. If a box is not needed leave it blank. (b) The standard enthalpy change for the following reaction is 108kJ at 298K. XeF2(g)Xe(g)+F2(g) What is the standard heat of formation of XeF2(g) ? kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


