Question: Tutored Practice Problem 15.3.4 Use K and initial concentrations to calculate equilibrium concentrations. Consider the equilibrium system involving the decomposition of methylene chloride. 2CH2Cl2(g)CH4(g)+CCl4(g)K=[CH2Cl2]2[CH4][CCl4]=9.09at358K A
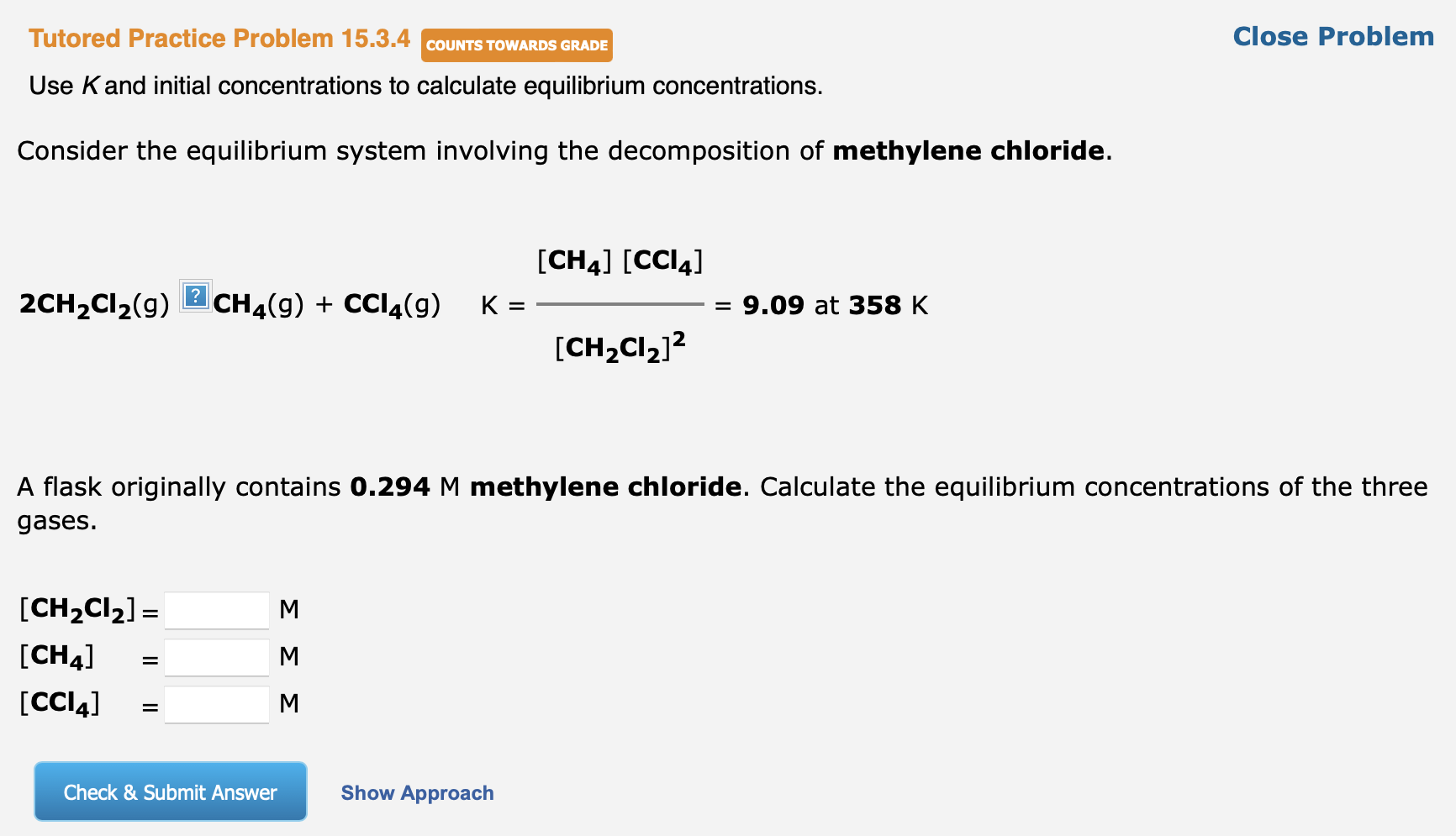
Tutored Practice Problem 15.3.4 Use K and initial concentrations to calculate equilibrium concentrations. Consider the equilibrium system involving the decomposition of methylene chloride. 2CH2Cl2(g)CH4(g)+CCl4(g)K=[CH2Cl2]2[CH4][CCl4]=9.09at358K A flask originally contains 0.294M methylene chloride. Calculate the equilibrium concentrations of the three gases. [CH2Cl2]=[CH4]=[CCl4]=MMM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


