Question: Empirical Formulas from Percent Composition Tutored Practice Problem 3.2.3 Use percent composition to determine an empirical formula. A compound is found to contain S,O, and
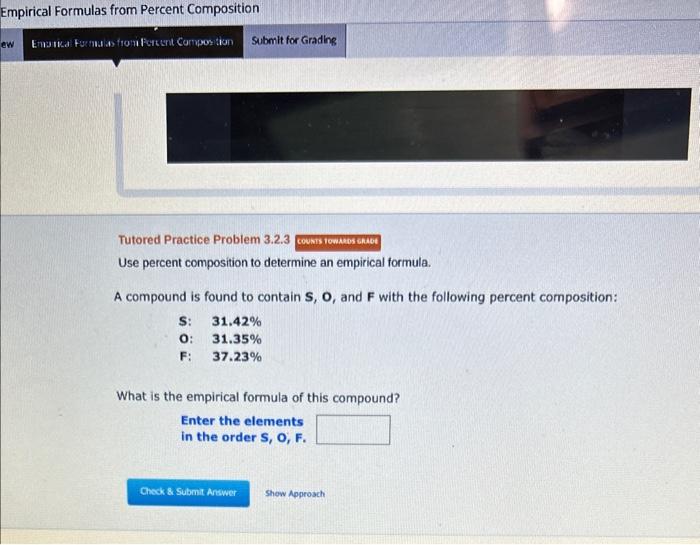
Empirical Formulas from Percent Composition Tutored Practice Problem 3.2.3 Use percent composition to determine an empirical formula. A compound is found to contain S,O, and F with the following percent composition: S:O:F:31.42%31.35%37.23% What is the empirical formula of this compound? Enter the elements in the order S, O, F
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


