Question: Tutored Practice-Problem 8.3.2 Eansw vowerwave Identify limiting reactants (maximum product method). Close Problem Consider the reaction of sodium with carbon and ammonia. 2Na(s)+2C(s)+2NH3(g)2NaCN(s)+3H2(g) Determine the
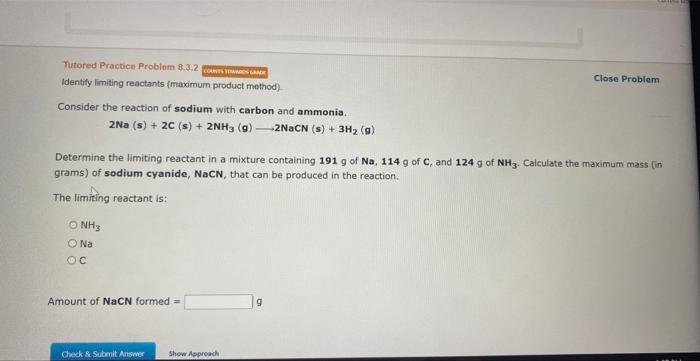
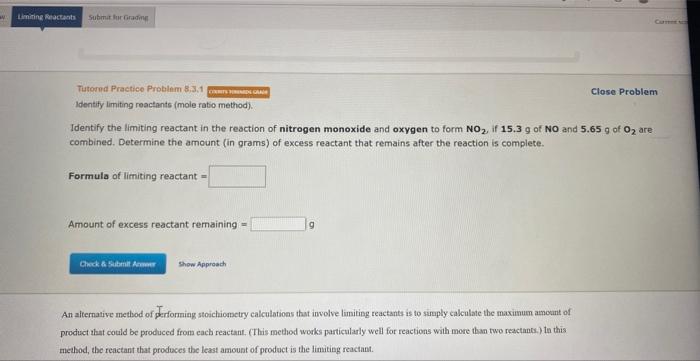
Tutored Practice-Problem 8.3.2 Eansw vowerwave Identify limiting reactants (maximum product method). Close Problem Consider the reaction of sodium with carbon and ammonia. 2Na(s)+2C(s)+2NH3(g)2NaCN(s)+3H2(g) Determine the limiting reactant in a mixture containing 191g of Na,114g of C, and 124g of NH3. Calculate the maximum mass (in grams) of sodium cyanide, NaCN, that can be produced in the reaction. The limiting reactant is: NH3 Na C Amount of NaCN formed = 9 Tutornd Practice Problem 8,3,1 Close Problem Identify limiting reactants (mole ratio method). Identify the limiting reactant in the reaction of nitrogen monoxide and oxygen to form NO2, if 15.3g of NO and 5.65g of O2 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete. Formula of limiting reactant = Amount of excess reactant remaining = An alternative method of performing stoichiometry calculations that involve limiting reactants is to simply calculate the maximum amoant of product that could be produced from each reactant, (This method works particularly well for reactions with more than two reactance.) ta this method, the reactant that prodoces the least amount of product is the limiting reactant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


