Question: Tutored Practice Problem 3.4.2 COUNTS TOWARDS GRADE Close Problem Identify limiting reactants (maximum product method). Consider the reaction of sodium with carbon and ammonia. 2Na
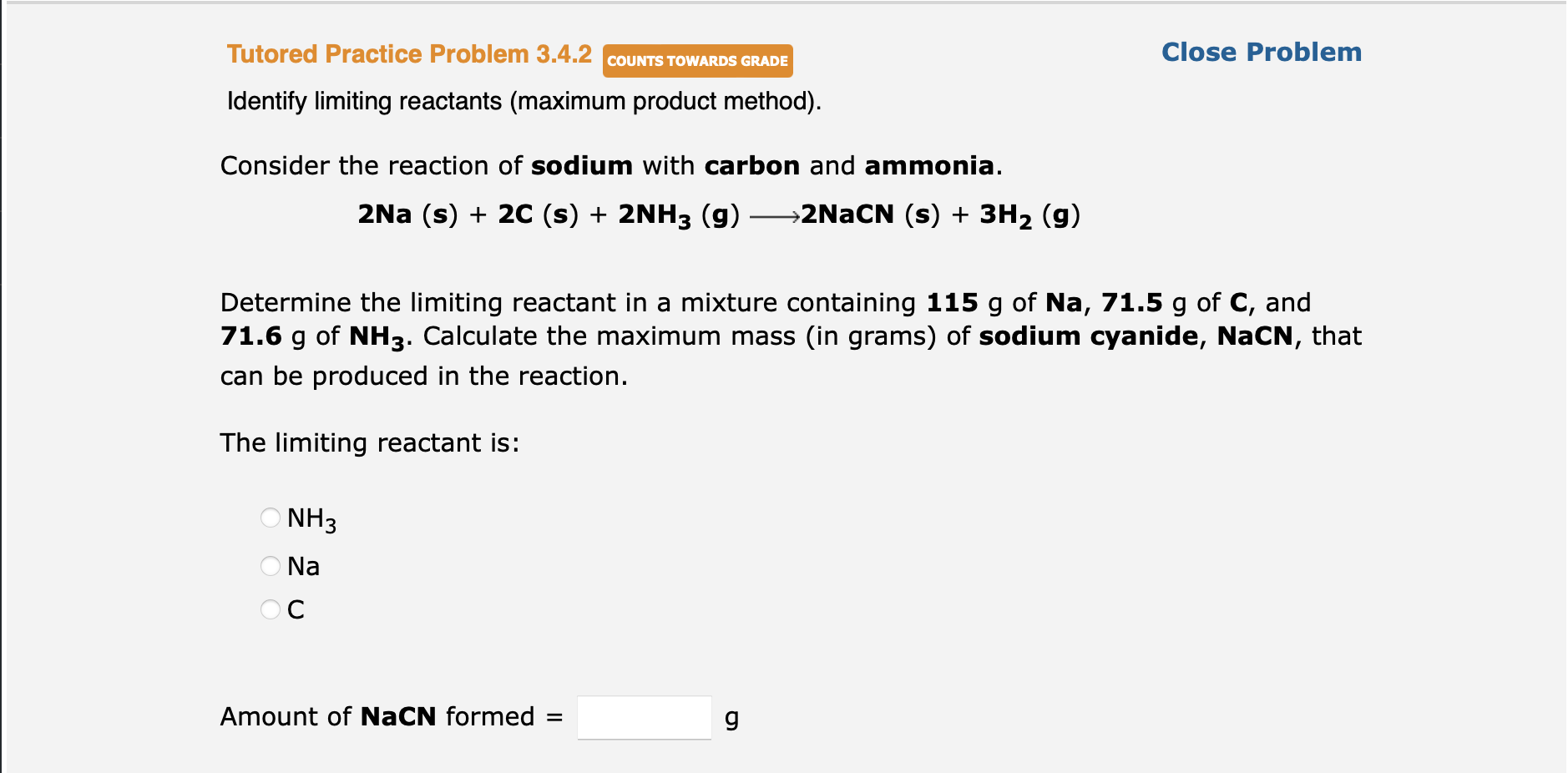
Tutored Practice Problem 3.4.2 COUNTS TOWARDS GRADE Close Problem Identify limiting reactants (maximum product method). Consider the reaction of sodium with carbon and ammonia. 2Na (s) + 2C (s) + 2NH3 (g) >2NaCN (s) + 3H2 (g) Determine the limiting reactant in a mixture containing 115 g of Na, 71.5 g of C, and 71.6 g of NH3. Calculate the maximum mass (in grams) of sodium cyanide, NaCN, that can be produced in the reaction. . The limiting reactant is: NH3 Na OC Amount of NaCN formed = g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


