Question: Tutorial 15: Diffusion-reaction in spherical coordinates A chemical dimerization reaction in the gas-phase takes place on the surface of metal-coated spherical beads (5 mm in
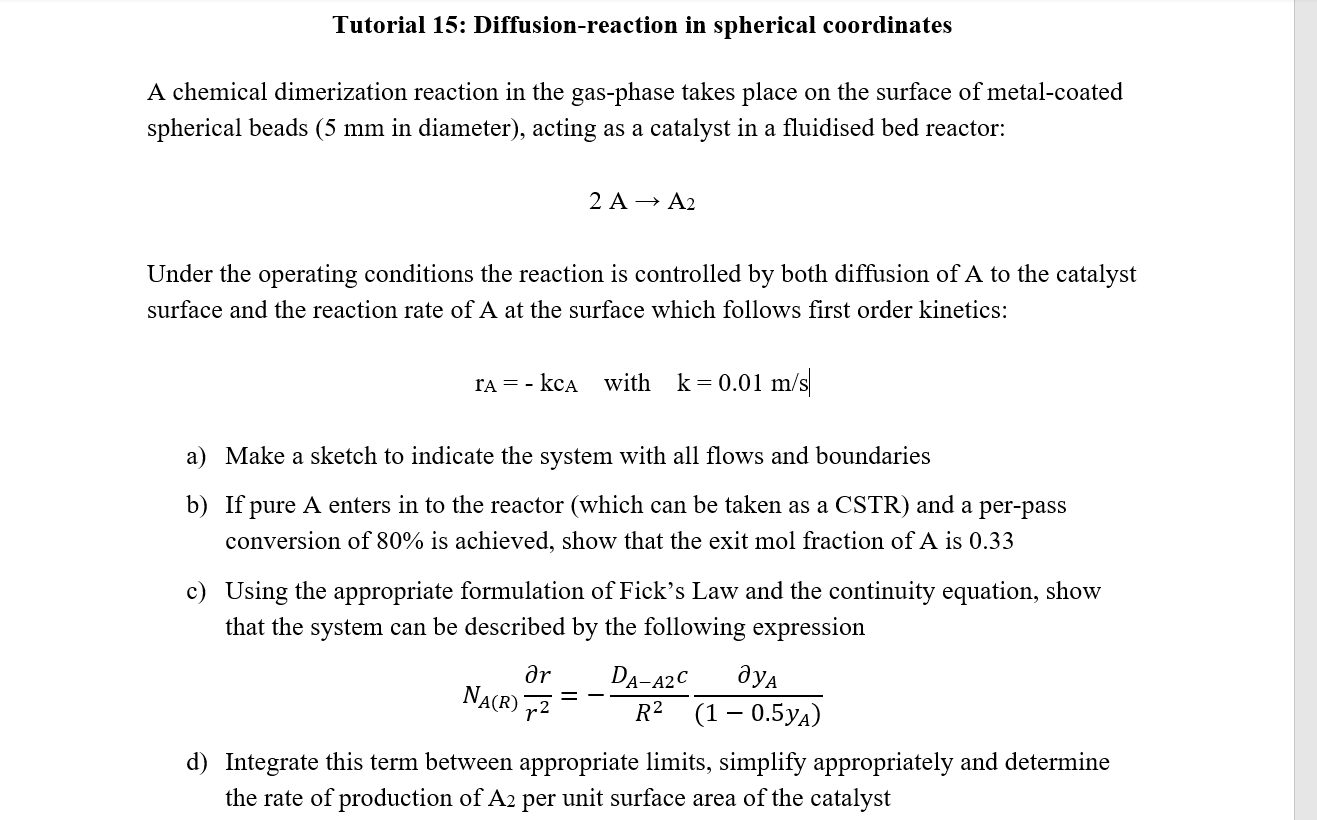
Tutorial 15: Diffusion-reaction in spherical coordinates A chemical dimerization reaction in the gas-phase takes place on the surface of metal-coated spherical beads (5 mm in diameter), acting as a catalyst in a fluidised bed reactor: 2 A A2 Under the operating conditions the reaction is controlled by both diffusion of A to the catalyst surface and the reaction rate of A at the surface which follows first order kinetics: ra = - kca with k=0.01 m/s a) Make a sketch to indicate the system with all flows and boundaries b) If pure A enters in to the reactor (which can be taken as a CSTR) and a per-pass conversion of 80% is achieved, show that the exit mol fraction of A is 0.33 c) Using the appropriate formulation of Fick's Law and the continuity equation, show that the system can be described by the following expression ar DA-A2C R2 (1 0.5ya) NA(R) FZ = d) Integrate this term between appropriate limits, simplify appropriately and determine the rate of production of A2 per unit surface area of the catalyst
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


