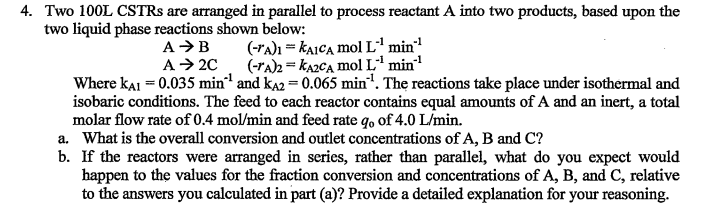
Question: Two 1 0 0 L CSTRs are arranged in parallel to process reactant A into two products, based upon the two liquid phase reactions shown
Two L CSTRs are arranged in parallel to process reactant A into two products, based upon the
two liquid phase reactions shown below:
min
min
Where and The reactions take place under isothermal and
isobaric conditions. The feed to each reactor contains equal amounts of A and an inert, a total
molar flow rate of and feed rate of
a What is the overall conversion and outlet concentrations of and
b If the reactors were arranged in series, rather than parallel, what do you expect would
happen to the values for the fraction conversion and concentrations of and relative
to the answers you calculated in part a Provide a detailed explanation for your reasoning.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


