Question: Two 20.0 g ice cubes at 17.0 C are placed into 205 g of water at 25.0 C. Assuming no energy is transferred to or
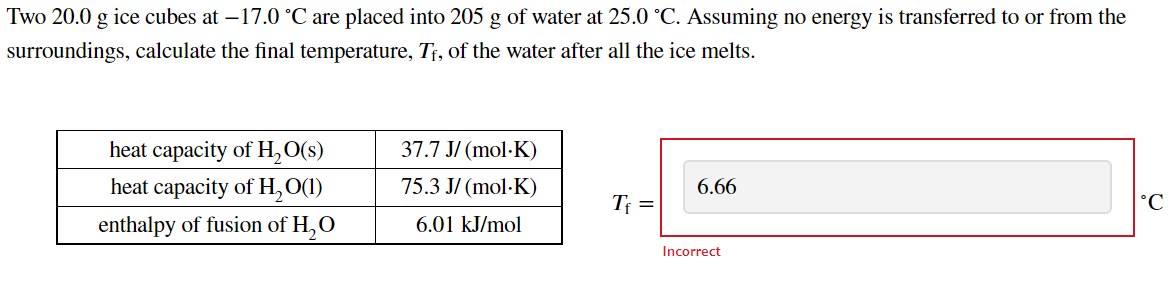
Two 20.0 g ice cubes at 17.0 C are placed into 205 g of water at 25.0 C. Assuming no energy is transferred to or from the g g surroundings, calculate the final temperature, Tf, of the water after all the ice melts. heat capacity of H2O(s) heat capacity of H2O(1) enthalpy of fusion of H, O 37.7 J/(mol.K) 75.3 J/mol.K) 6.66 Ti = C 6.01 kJ/mol Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


