Question: Two different emission processes are shown in the energy level diagram. Which transition results in the emission of a photon with higher energy? n=
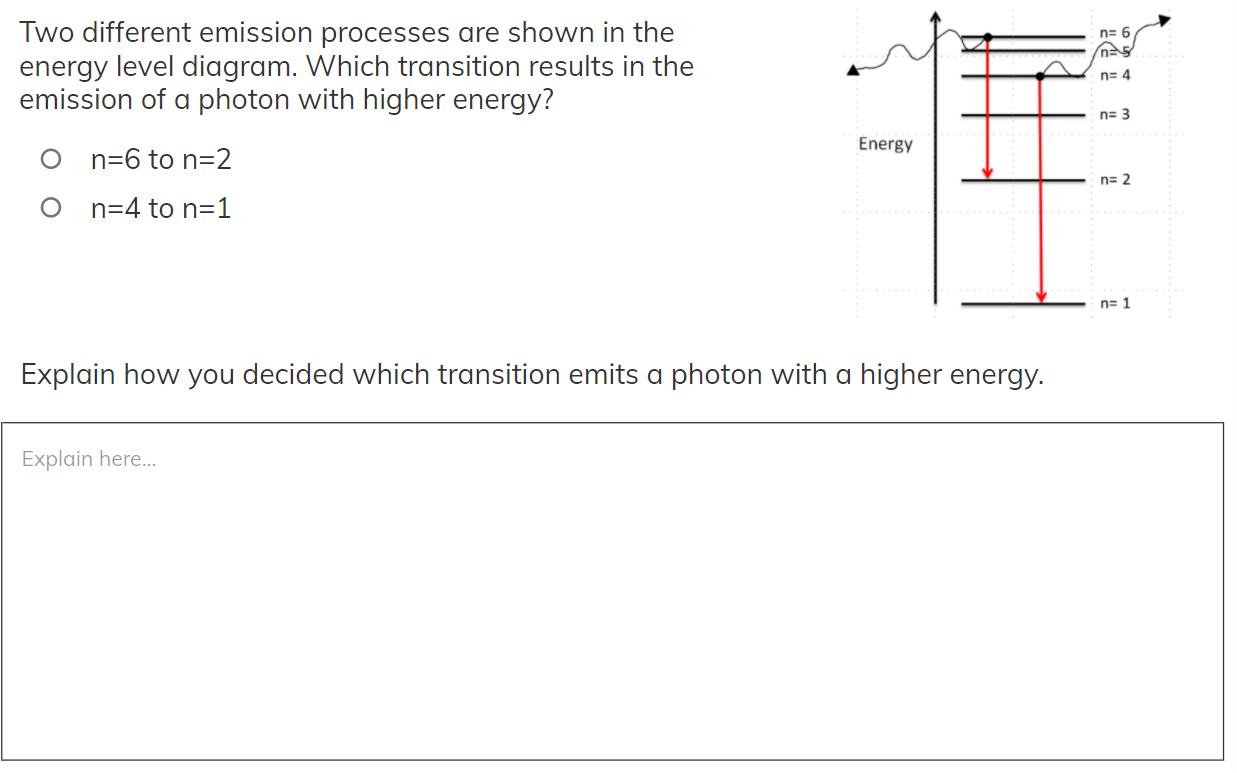
Two different emission processes are shown in the energy level diagram. Which transition results in the emission of a photon with higher energy? n= 6 n= 4 n= 3 Energy O n=6 to n=2 n= 2 O n=4 to n=1 n= 1 Explain how you decided which transition emits a photon with a higher energy. Explain here..
Step by Step Solution
3.59 Rating (153 Votes )
There are 3 Steps involved in it
As we know when an electron jumps from higher energy state n1 to lower one ... View full answer

Get step-by-step solutions from verified subject matter experts


