Question: Two experts have already tried and are currently getting it wrong I hope these arent blurry this time, i made sure they were good estabttande
Two experts have already tried and are currently getting it wrong 

I hope these arent blurry this time, i made sure they were good
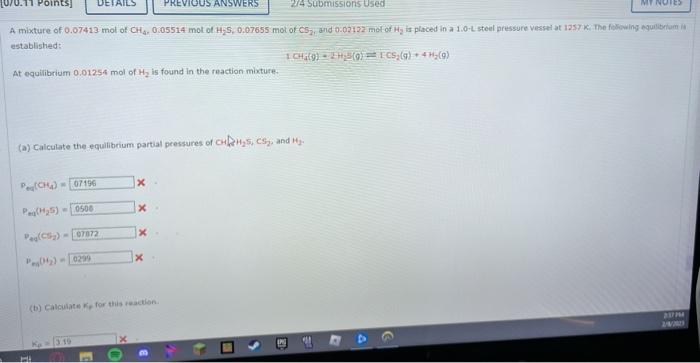
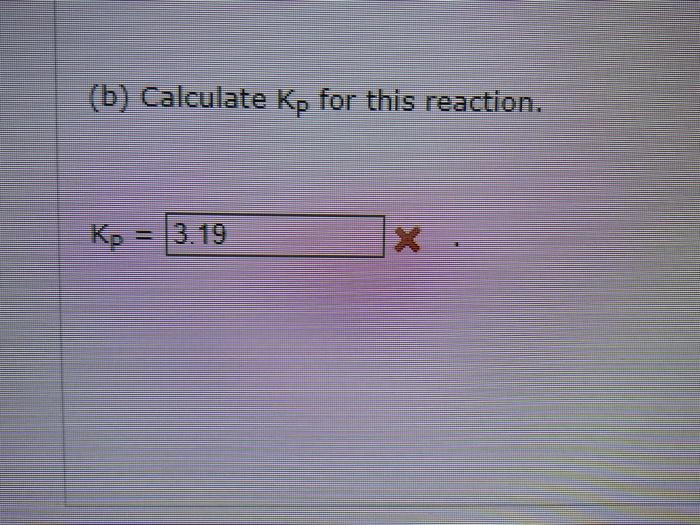
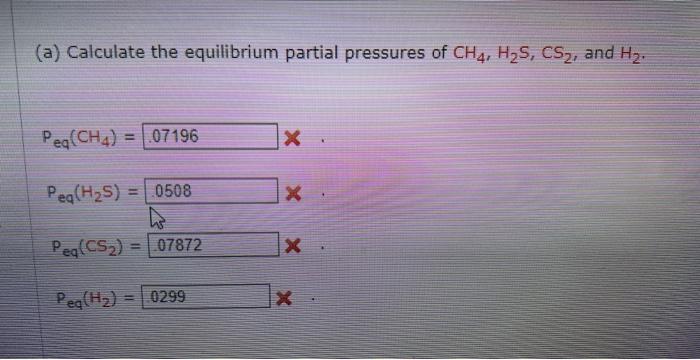
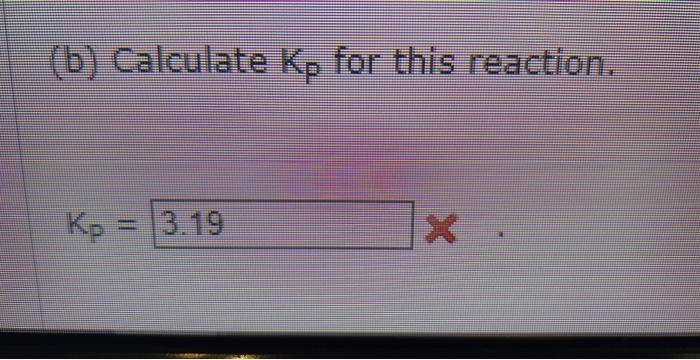
estabttande (a) Calculate the equilibrium partial pressures of CH4,H2S,CS2, and H2. Peq(CH4)= Peq(H2S)= Peq(C2)= Peqeq(H2)= (b) Calculate Kp for this reaction. established: 1CH1(9)1H1(g)=Ics2(9)+4H2(9) At equilibrium 0.01254mol of H2 is found in the reaction mixture. (a) Calculate the equilibciam partial pressures of CH1H25,CS2 and H2 : P.([CH4)=Pmi((H25)=Pad((Crta))=ie9(i2)= (b) Calculate kif for this reaction. (b) Calculate Kp for this reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


