Question: Two flask of equal volume have been joined by a narrow tube of negligible volume. Initially both flasks are at 300K containing 0.60 mole
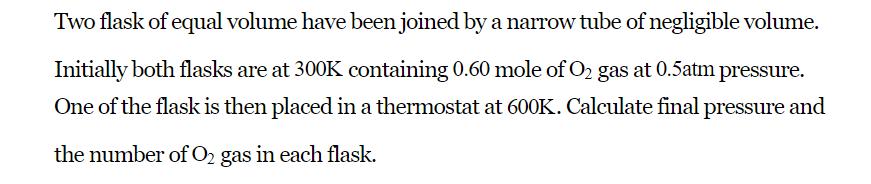
Two flask of equal volume have been joined by a narrow tube of negligible volume. Initially both flasks are at 300K containing 0.60 mole of O gas at 0.5atm pressure. One of the flask is then placed in a thermostat at 600K. Calculate final pressure and the number of O gas in each flask.
Step by Step Solution
★★★★★
3.50 Rating (167 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

AnswerIdeal gas law PVnRT P1Vn1RT Volume is constant 05V106R300 V360R in 2nd case ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


