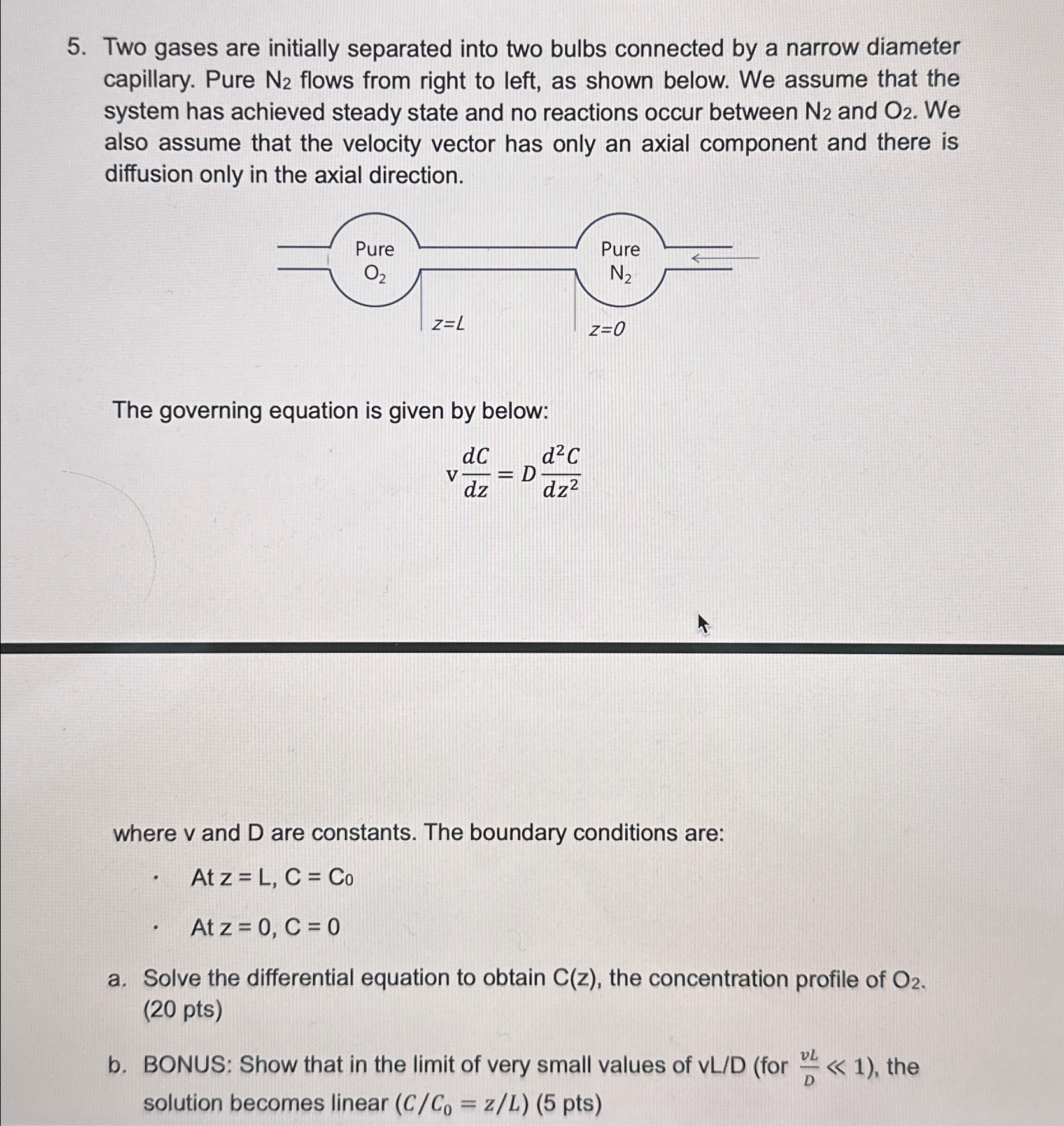
Question: Two gases are initially separated into two bulbs connected by a narrow diameter capillary. Pure N 2 flows from right to left, as shown below.
Two gases are initially separated into two bulbs connected by a narrow diameter capillary. Pure flows from right to left, as shown below. We assume that the system has achieved steady state and no reactions occur between and We also assume that the velocity vector has only an axial component and there is diffusion only in the axial direction.
The governing equation is given by below:
where and are constants. The boundary conditions are:
At
At
a Solve the differential equation to obtain the concentration profile of pts
b BONUS: Show that in the limit of very small values of for the solution becomes linear
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


