Question: Two new students (C and D) both attempted separating 1.50g samples of this mixture discussed above. Both students used TLC and melting points to evaluate
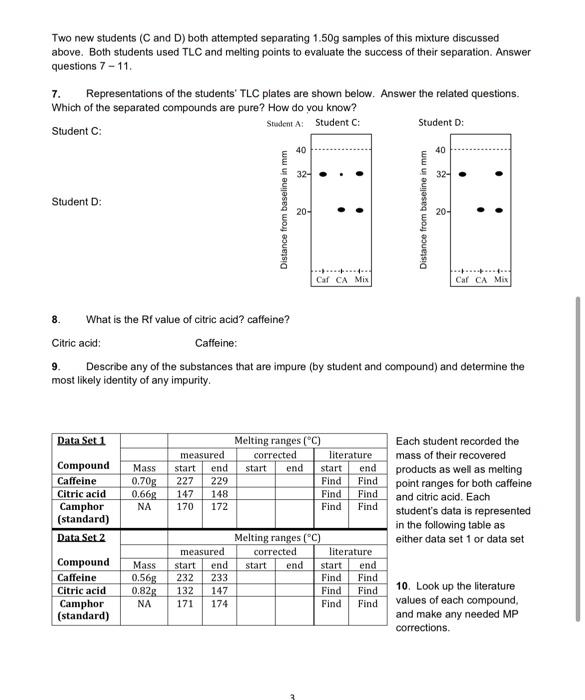
Two new students (C and D) both attempted separating 1.50g samples of this mixture discussed above. Both students used TLC and melting points to evaluate the success of their separation. Answer questions 7-11. 7. Representations of the students' TLC plates are shown below. Answer the related questions. Which of the separated compounds are pure? How do you know? Student C Student A: Student Student D: 40 40 32- . . 32- Student D: Distance from baseline in mm 20- . Distance from baseline in mm 20- . .........4 Caf CA Mix Cal CA Mix 8. What is the Rf value of citric acid? caffeine? Citric acid Caffeine: Describe any of the substances that are impure (by student and compound) and determine the most likely identity of any impurity. Data Set 1 Mass 0.70g 0.66% NA measured start end 229 147 148 170 172 Melting ranges (C) corrected literature start end start end Find Find Find Find Find Find 227 CH Compound Caffeine Citric acid Camphor (standard) Data Set 2 Compound Caffeine Citric acid Camphor (standard) Each student recorded the mass of their recovered products as well as melting point ranges for both caffeine and citric acid. Each student's data is represented in the following table as either data set 1 or data set Mass 0.568 0.828 NA Melting ranges (C) measured corrected literature start end start end start end 232 233 Find Find 132 147 Find Find 171 174 Find Find TI 10. Look up the literature values of each compound, and make any needed MP corrections
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


