Question: Two vessels separately contain two ideal gases A and B at the same temperature. The pressure of A being twice that of B. Under
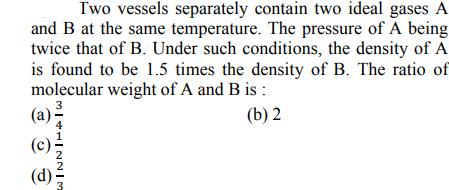
Two vessels separately contain two ideal gases A and B at the same temperature. The pressure of A being twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weight of A and B is : 3 (b) 2 (a)- IHINNIM (d).
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


