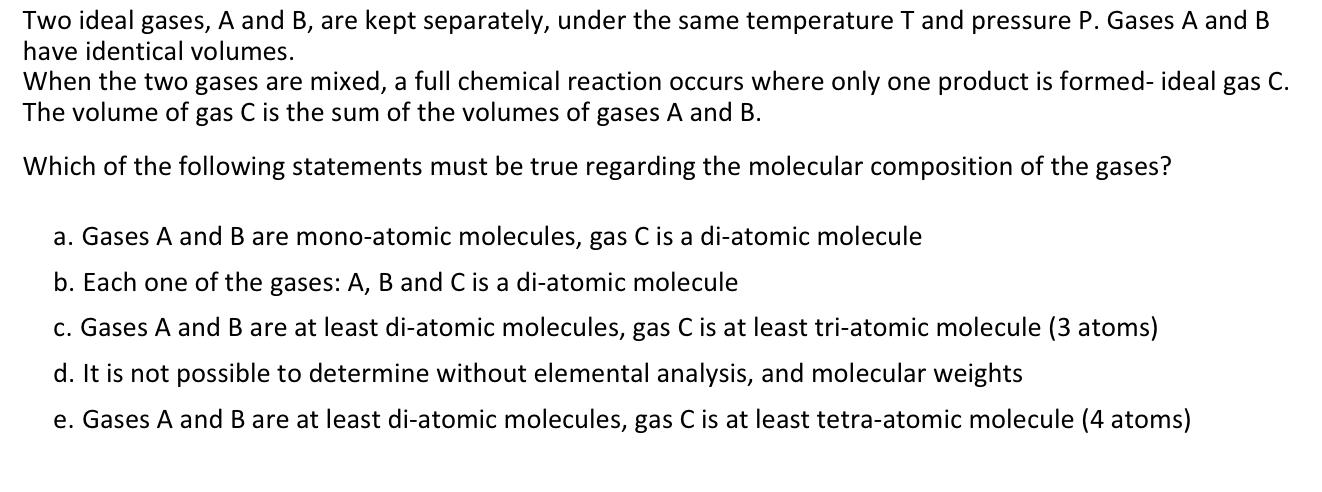
Question: Two ideal gases, A and B , are kept separately, under the same temperature T and pressure P . Gases A and B have identical
Two ideal gases, A and are kept separately, under the same temperature and pressure Gases A and have identical volumes.
When the two gases are mixed, a full chemical reaction occurs where only one product is formedideal gas The volume of gas is the sum of the volumes of gases A and
Which of the following statements must be true regarding the molecular composition of the gases?
a Gases A and are monoatomic molecules, gas is a diatomic molecule
b Each one of the gases: A B and C is a diatomic molecule
c Gases A and B are at least diatomic molecules, gas C is at least triatomic molecule atoms
d It is not possible to determine without elemental analysis, and molecular weights
e Gases A and B are at least diatomic molecules, gas C is at least tetraatomic molecule atoms
Correct answer is b how to do this, totally have no idea
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


