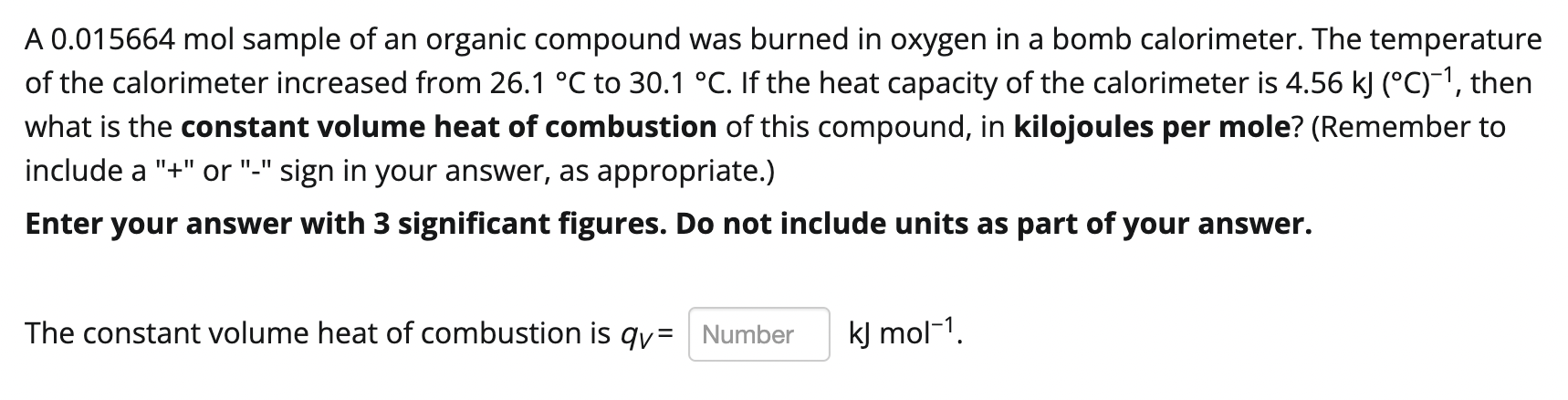
Question: Type or paste question here a A 0.015664 mol sample of an organic compound was burned in oxygen in a bomb calorimeter. The temperature of
 Type or paste question here
Type or paste question here
a A 0.015664 mol sample of an organic compound was burned in oxygen in a bomb calorimeter. The temperature of the calorimeter increased from 26.1 C to 30.1 C. If the heat capacity of the calorimeter is 4.56 kJ (C)-?, then what is the constant volume heat of combustion of this compound, in kilojoules per mole? (Remember to include a "+" or "-" sign in your answer, as appropriate.) Enter your answer with 3 significant figures. Do not include units as part of your answer. a The constant volume heat of combustion is qv= Number kJ mol-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


