Question: TYPE YOUR QUESTION HERE Sample Problem No. 3 A 0.9092 g sample of a wheat flour was analyzed by the Kjeldahl procedure. The liberated ammonia
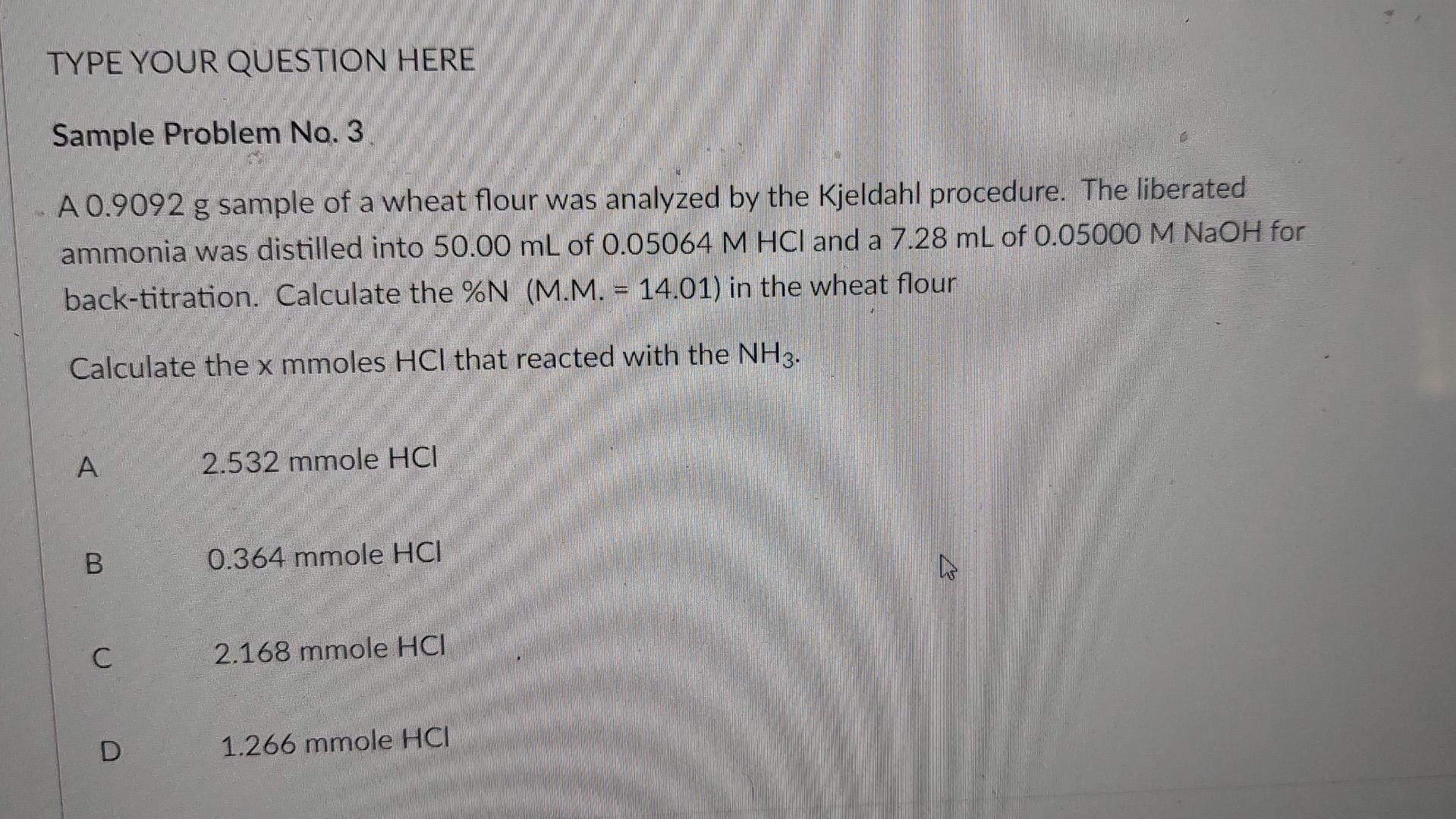
TYPE YOUR QUESTION HERE Sample Problem No. 3 A 0.9092 g sample of a wheat flour was analyzed by the Kjeldahl procedure. The liberated ammonia was distilled into 50.00 mL of 0.05064 M HCl and a 7.28 mL of 0.05000 M NaOH for back-titration. Calculate the %N (M.M. - 14.01) in the wheat flour Calculate the x mmoles HCl that reacted with the NH3. A 2.532 mmole HCI B 0.364 mmole HCI C C 2.168 mmole HCI D 1.266 mmole HCI
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


