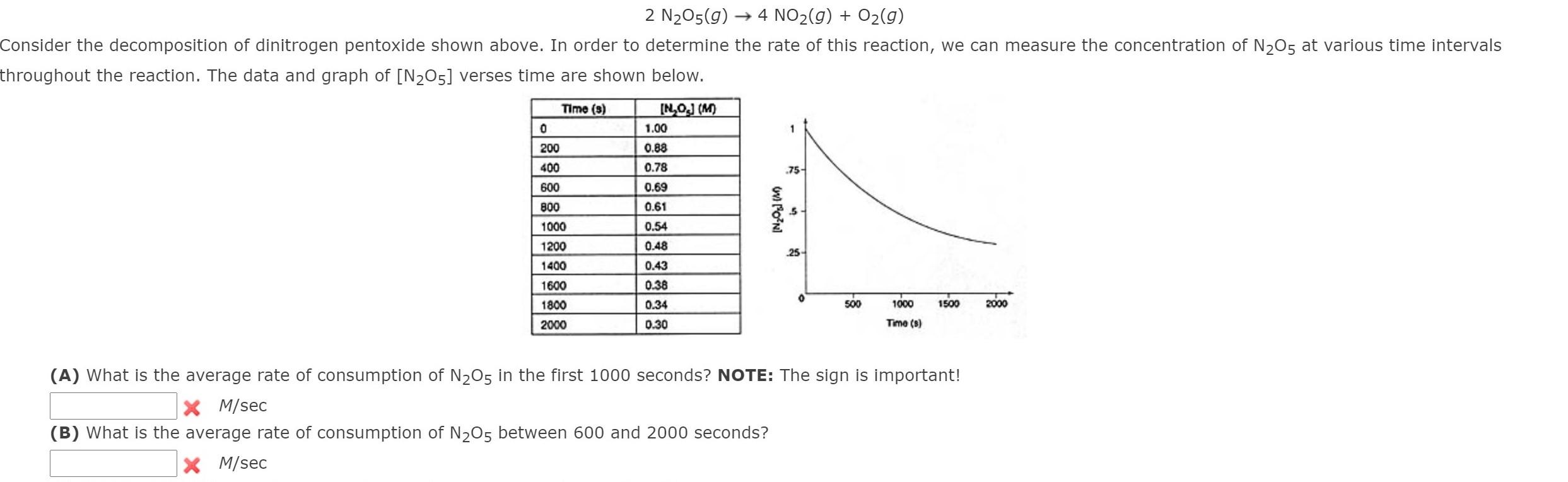
Question: undefined 2 N205(g) 4 NO2(g) + O2(g) Consider the decomposition of dinitrogen pentoxide shown above. In order to determine the rate of this reaction, we
 undefined
undefined
2 N205(g) 4 NO2(g) + O2(g) Consider the decomposition of dinitrogen pentoxide shown above. In order to determine the rate of this reaction, we can measure the concentration of N205 at various time intervals throughout the reaction. The data and graph of [N205] verses time are shown below. Time (3) INOJ (M) 1.00 0 200 0.88 0.78 400 .75 0.69 0.61 wilo Ni .5 0.54 600 800 1000 1200 1400 1600 0.48 25 0.43 0.38 0.34 1800 500 1000 1500 2000 2000 0.30 Time (8) (A) What is the average rate of consumption of N205 in the first 1000 seconds? NOTE: The sign is important! X M/sec (B) What is the average rate of consumption of N205 between 600 and 2000 seconds? M/sec
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


