Question: Unit 3- SCH4U Worksheet Dr. sarshar Enthalpy of Formation: Problem-Solving 1. In the early days of automobiles, illumination at night was provided by burning acetylene(or
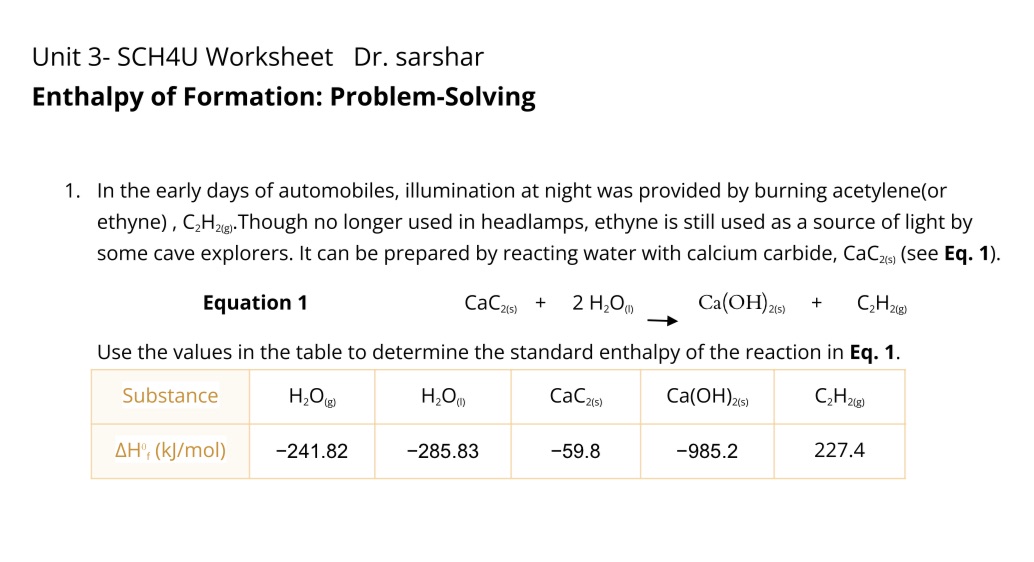
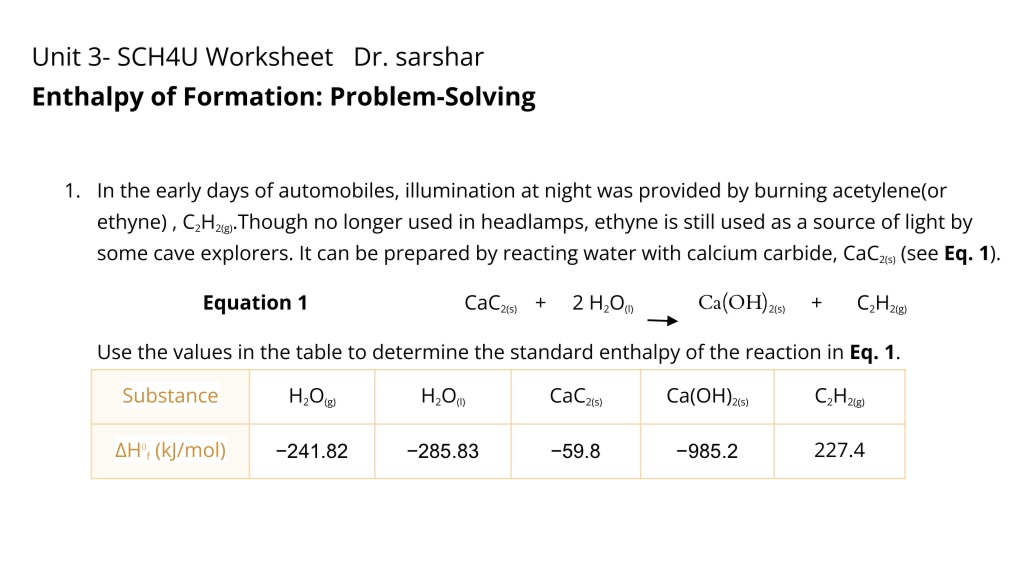
Unit 3- SCH4U Worksheet Dr. sarshar Enthalpy of Formation: Problem-Solving 1. In the early days of automobiles, illumination at night was provided by burning acetylene(or ethyne) , C2H2). Though no longer used in headlamps, ethyne is still used as a source of light by some cave explorers. It can be prepared by reacting water with calcium carbide, CaCas) (see Eq. 1). Equation 1 CaC2(5) + 2 H20( Ca(OH)2(6) + C2 H 2(8) Use the values in the table to determine the standard enthalpy of the reaction in Eq. 1. Substance H2O (F) CaC2(5) Ca(OH)2(5) C 2 H 2(8 ) AH', (kj/mol) -241.82 -285.83 -59.8 -985.2 227.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


